Rapid tumor DNA analysis of cerebrospinal fluid accelerates treatment of central nervous system lymphoma
- PMID: 38776489
- PMCID: PMC11406186
- DOI: 10.1182/blood.2024023832
Rapid tumor DNA analysis of cerebrospinal fluid accelerates treatment of central nervous system lymphoma
Abstract
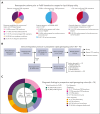
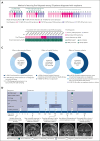
Delays and risks associated with neurosurgical biopsies preclude timely diagnosis and treatment of central nervous system (CNS) lymphoma and other CNS neoplasms. We prospectively integrated targeted rapid genotyping of cerebrospinal fluid (CSF) into the evaluation of 70 patients with CNS lesions of unknown cause. Participants underwent genotyping of CSF-derived DNA using a quantitative polymerase chain reaction-based approach for parallel detection of single-nucleotide variants in the MYD88, TERT promoter, IDH1, IDH2, BRAF, and H3F3A genes within 80 minutes of sample acquisition. Canonical mutations were detected in 42% of patients with neoplasms, including cases of primary and secondary CNS lymphoma, glioblastoma, IDH-mutant brainstem glioma, and H3K27M-mutant diffuse midline glioma. Genotyping results eliminated the need for surgical biopsies in 7 of 33 cases (21.2%) of newly diagnosed neoplasms, resulting in significantly accelerated initiation of disease-directed treatment (median, 3 vs 12 days; P = .027). This assay was then implemented in a Clinical Laboratory Improvement Amendments environment, with 2-day median turnaround for diagnosis of CNS lymphoma from 66 patients across 4 clinical sites. Our study prospectively demonstrates that targeted rapid CSF genotyping influences oncologic management for suspected CNS tumors.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: D.P.C. and G.M.S. hold a patent pertaining to the use of peptide and locked nucleic acids as used in this study during the amplification of target DNA sequences (patent number US20170369939A1). Outside the scope of this work, the authors report the following. R.G. serves as a paid consultant for Idorsia, Inc, and Mosaic Research Management; is an advisory board member of Braintale, Inc, and the University of Wisconsin’s computed tomography board; provided expert testimony to Mary Hitchcock Memorial Hospital; has received speaker honoraria from Siemens; provided legal consulting to the Harvard Medical Institutions Risk Management Foundation; and received research funding from Samsung and the US National Institutes of Health. N.W. has received royalties from Wolters Kluwer and research funding from Merck, and was previously on the advisory board of Seattle Genetics (ended 2021). P.K.B. has consulted for Merck, Genentech-Roche, Eli Lilly, Tesaro, ElevateBio, Dantari, Voyager Therapeutics, SK Life Sciences, Pfizer, ACI, Atavistik, Sintetica, Kazia, MPM, CraniUS, Axiom, and InCephalo; serves on the Scientific Advisory Board for Kazia and CraniUS; has received grant/research support (to institution) from Merck, GlaxoSmithKline, AstraZeneca, Kazia, Genentech-Roche, Eli Lilly, Mirati, Bristol Myers Squibb, and Kinnate; and has received speaker’s honoraria from Merck, Genentech-Roche, Eli Lilly, and Medscape. D.A.F. is a shareholder for Eli Lilly. B.V.N. has consulted for Robeaute, BK Medical, Merck, BrainLab, Zeta, and Enclear. He has received grant funding from the US National Institutes of Health. T.T.B. has received publishing royalties from UpToDate, Inc, and Oxford University Press; and has received research institutional grant/research support related to a clinical trial (to Brigham and Women’s Hospital) from ONO Pharmaceuticals. L.L.R. has received honoraria from PeerView, Medscape, and Clinical Care Options; and has received consulting honoraria from AbbVie, Personal Genome Diagnostics, Bristol Myers Squibb, Loxo Oncology at Lilly, Amgen, Merck, AstraZeneca, Sanofi-Genzyme, and EMD Serono. J.T.J. holds equity in Navio Theragnostics, Akeila Bio, and The Doctor Lounge; and has participated in paid consulting from Navio Theragnostics, Recursion Pharmaceuticals, Alexion Pharmaceuticals, Springworks Pharmaceuticals, Merck Pharmaceuticals, Children’s Tumor Foundation, CEC Oncology, and Elsevier. J.D. is a consultant for Amgen, Blue Earth Diagnostics, and Unum Therapeutics; and has received research support from Novartis and publishing royalties from Wolters for UpToDate, Inc. M.M. consults for Bayer, DelveBio, Interline, and Isabl; holds equity in DelveBio, Interline, and Isabl; is an inventor of patents licensed to LabCorp and Bayer; and receives research funding from Bayer and Janssen. D.P.C. has consulted for the Massachusetts Institute of Technology, Advise Connect Inspire, Lilly, GlaxoSmithKline, Iconovir, Boston Pharmaceuticals, and Boston Scientific; serves on the advisory board of Pyramid Biosciences, which includes an equity interest; and has received honoraria and travel reimbursement from Merck for invited lectures, and from the US National Institutes of Health and Department of Defense for clinical trial and grant review. B.S.C. is a consultant for Koh Young. The remaining authors declare no competing financial interests.
Figures


References
-
- Omuro AM, Leite CC, Mokhtari K, Delattre JY. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5(11):937–948. - PubMed
-
- Velasco R, Mercadal S, Vidal N, et al. Diagnostic delay and outcome in immunocompetent patients with primary central nervous system lymphoma in Spain: a multicentric study. J Neurooncol. 2020;148(3):545–554. - PubMed
-
- Lau BL, Vijian K, Liew DNS, Wong ASH. Factors affecting diagnostic yield in stereotactic biopsy for brain lesions: a 5-year single-center series. Neurosurg Rev. 2022;45(2):1473–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

