Therapeutic avenues in bone repair: Harnessing an anabolic osteopeptide, PEPITEM, to boost bone growth and prevent bone loss
- PMID: 38776873
- PMCID: PMC11148860
- DOI: 10.1016/j.xcrm.2024.101574
Therapeutic avenues in bone repair: Harnessing an anabolic osteopeptide, PEPITEM, to boost bone growth and prevent bone loss
Erratum in
-
Therapeutic avenues in bone repair: Harnessing an anabolic osteopeptide, PEPITEM, to boost bone growth and prevent bone loss.Cell Rep Med. 2024 Aug 20;5(8):101657. doi: 10.1016/j.xcrm.2024.101657. Epub 2024 Jul 9. Cell Rep Med. 2024. PMID: 38986622 Free PMC article. No abstract available.
Abstract
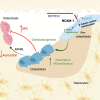
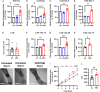
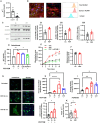
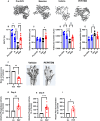
The existing suite of therapies for bone diseases largely act to prevent further bone loss but fail to stimulate healthy bone formation and repair. We describe an endogenous osteopeptide (PEPITEM) with anabolic osteogenic activity, regulating bone remodeling in health and disease. PEPITEM acts directly on osteoblasts through NCAM-1 signaling to promote their maturation and formation of new bone, leading to enhanced trabecular bone growth and strength. Simultaneously, PEPITEM stimulates an inhibitory paracrine loop: promoting osteoblast release of the decoy receptor osteoprotegerin, which sequesters RANKL, thereby limiting osteoclast activity and bone resorption. In disease models, PEPITEM therapy halts osteoporosis-induced bone loss and arthritis-induced bone damage in mice and stimulates new bone formation in osteoblasts derived from patient samples. Thus, PEPITEM offers an alternative therapeutic option in the management of diseases with excessive bone loss, promoting an endogenous anabolic pathway to induce bone remodeling and redress the imbalance in bone turnover.
Keywords: NCAM-1; OPG; PEPITEM; b-catenin; bone; bone mineral density; osteoblast; osteoclast; osteoporosis; rheumatoid arthritis.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.M.M., G.E.R., A.J.I., and M.C. hold patents on the use of PEPITEM in bone diseases and other inflammatory conditions (US9597368B2; US9839671B; EP2802342; JP6055845; CN104168910B; US-2021-0100870-A1; GB2301317.0; PCT/GB2023/052013). H.M.M. and A.J.I. have received funding from Roche.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

