A novel gain-of-function phosphorylation site modulates PTPN22 inhibition of TCR signaling
- PMID: 38777143
- PMCID: PMC11237943
- DOI: 10.1016/j.jbc.2024.107393
A novel gain-of-function phosphorylation site modulates PTPN22 inhibition of TCR signaling
Abstract
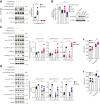
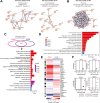
Protein tyrosine phosphatase nonreceptor type 22 (PTPN22) is encoded by a major autoimmunity gene and is a known inhibitor of T cell receptor (TCR) signaling and drug target for cancer immunotherapy. However, little is known about PTPN22 posttranslational regulation. Here, we characterize a phosphorylation site at Ser325 situated C terminal to the catalytic domain of PTPN22 and its roles in altering protein function. In human T cells, Ser325 is phosphorylated by glycogen synthase kinase-3 (GSK3) following TCR stimulation, which promotes its TCR-inhibitory activity. Signaling through the major TCR-dependent pathway under PTPN22 control was enhanced by CRISPR/Cas9-mediated suppression of Ser325 phosphorylation and inhibited by mimicking it via glutamic acid substitution. Global phospho-mass spectrometry showed Ser325 phosphorylation state alters downstream transcriptional activity through enrichment of Swi3p, Rsc8p, and Moira domain binding proteins, and next-generation sequencing revealed it differentially regulates the expression of chemokines and T cell activation pathways. Moreover, in vitro kinetic data suggest the modulation of activity depends on a cellular context. Finally, we begin to address the structural and mechanistic basis for the influence of Ser325 phosphorylation on the protein's properties by deuterium exchange mass spectrometry and NMR spectroscopy. In conclusion, this study explores the function of a novel phosphorylation site of PTPN22 that is involved in complex regulation of TCR signaling and provides details that might inform the future development of allosteric modulators of PTPN22.
Keywords: PTPN22; T cell receptor; allosteric regulation; autoimmunity; catalysis; intrinsically disordered region; phosphoproteomics; phosphorylation; transcriptomics; tyrosine phosphatase.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Tonks N.K. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cel. Biol. 2006;7:833–846. - PubMed
-
- Hasegawa K., Martin F., Huang G., Tumas D., Diehl L., Chan A.C. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

