Nuclear receptor 4A1 (NR4A1) upregulated by n-butylidenephthalide via the mitogen-activated protein kinase (MAPK) pathway ameliorates drug-induced gingival enlargement
- PMID: 38777369
- PMCID: PMC11627475
- DOI: 10.1002/biof.2077
Nuclear receptor 4A1 (NR4A1) upregulated by n-butylidenephthalide via the mitogen-activated protein kinase (MAPK) pathway ameliorates drug-induced gingival enlargement
Abstract
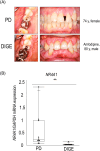
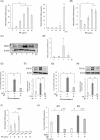
Drug-induced gingival enlargement (DIGE) is a side effect of ciclosporin, calcium channel blockers, and phenytoin. DIGE is a serious disease that leads to masticatory and esthetic disorders, severe caries, and periodontitis but currently has no standard treatment. We recently reported that nuclear receptor 4A1 (NR4A1) is a potential therapeutic target for DIGE. This study aimed to evaluate the therapeutic effects of n-butylidenephthalide (BP), which increases the expression of NR4A1, on DIGE. In this study, NR4A1 mRNA expression was analyzed in the patients with periodontal disease (PD) and DIGE. We evaluated the effect of BP on NR4A1 expression in gingival fibroblasts and in a DIGE mouse model. RNA sequencing (RNA-seq) was conducted to identify the mechanisms by which BP increases NR4A1 expression. The results showed that NR4A1 mRNA expression in the patients with DIGE was significantly lower than the patients with PD. BP suppressed the upregulation of COL1A1 expression, which was upregulated by TGF-β. BP also ameliorated gingival overgrowth in DIGE mice and reduced Col1a1 and Pai1 expression. BP also decreased Il1β mRNA expression in gingival tissue in DIGE. RNA-seq results showed an increase in the expression of several genes related to mitogen-activated protein kinase including DUSP genes in gingival fibroblasts stimulated by BP. Treatment with ERK and JNK inhibitors suppressed the BP-induced increase in NR4A1 expression. In addition, BP promoted the phosphorylation of ERK in gingival fibroblasts. In conclusion, BP increases NR4A1 expression in gingival fibroblasts through ERK and JNK signaling, demonstrating its potential as a preventive and therapeutic agent against DIGE.
Keywords: COL1A1; ERK; MAPK; NR4A1; drug‐induced gingival enlargement; n‐butylidenephthalide.
© 2024 The Authors. BioFactors published by Wiley Periodicals LLC on behalf of International Union of Biochemistry and Molecular Biology.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures





References
-
- Hatano S, Matsuda S, Okanobu A, Furutama D, Memida T, Kajiya M, et al. The role of nuclear receptor 4A1 (NR4A1) in drug‐induced gingival overgrowth. FASEB J. 2021;35:e21693. - PubMed
-
- Chen J, Jia J, Ma L, Li B, Qin Q, Qian J, et al. Nur77 deficiency exacerbates cardiac fibrosis after myocardial infarction by promoting endothelial‐to‐mesenchymal transition. J Cell Physiol. 2021;236:495–506. - PubMed
-
- Ma G, Chen F, Liu Y, Zheng L, Jiang X, Tian H, et al. Nur77 ameliorates age‐related renal tubulointerstitial fibrosis by suppressing the TGF‐β/Smads signaling pathway. FASEB J. 2022;36:e22124. - PubMed
-
- Palumbo‐Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor‐β signaling and fibrosis. Nat Med. 2015;21:150–158. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

