A multiomic characterization of the leukemia cell line REH using short- and long-read sequencing
- PMID: 38777370
- PMCID: PMC11111970
- DOI: 10.26508/lsa.202302481
A multiomic characterization of the leukemia cell line REH using short- and long-read sequencing
Abstract
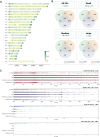
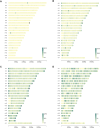
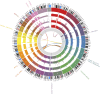
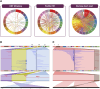
The B-cell acute lymphoblastic leukemia (ALL) cell line REH, with the t(12;21) ETV6::RUNX1 translocation, is known to have a complex karyotype defined by a series of large-scale chromosomal rearrangements. Taken from a 15-yr-old at relapse, the cell line offers a practical model for the study of pediatric B-ALL. In recent years, short- and long-read DNA and RNA sequencing have emerged as a complement to karyotyping techniques in the resolution of structural variants in an oncological context. Here, we explore the integration of long-read PacBio and Oxford Nanopore whole-genome sequencing, IsoSeq RNA sequencing, and short-read Illumina sequencing to create a detailed genomic and transcriptomic characterization of the REH cell line. Whole-genome sequencing clarified the molecular traits of disrupted ALL-associated genes including CDKN2A, PAX5, BTG1, VPREB1, and TBL1XR1, as well as the glucocorticoid receptor NR3C1 Meanwhile, transcriptome sequencing identified seven fusion genes within the genomic breakpoints. Together, our extensive whole-genome investigation makes high-quality open-source data available to the leukemia genomics community.
© 2024 Lysenkova Wiklander et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
