Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis
- PMID: 38777573
- PMCID: PMC11458363
- DOI: 10.1136/gutjnl-2023-331447
Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis
Abstract
Objective: Alcohol use in metabolic dysfunction-associated steatohepatitis (MASH) is associated with an increased risk of fibrosis and liver-related death. Here, we aimed to identify a mechanism through which repeated alcohol binges exacerbate liver injury in a high fat-cholesterol-sugar diet (MASH diet)-induced model of MASH.
Design: C57BL/6 mice received either chow or the MASH diet for 3 months with or without weekly alcohol binges. Neutrophil infiltration, neutrophil extracellular traps (NETs) and fibrosis were evaluated.
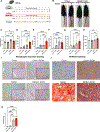
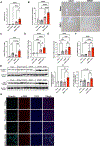
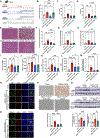
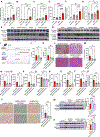
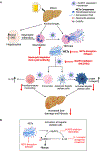
Results: We found that alcohol binges in MASH increase liver injury and fibrosis. Liver transcriptomic profiling revealed differential expression of genes involved in extracellular matrix reorganisation, neutrophil activation and inflammation compared with alcohol or the MASH diet alone. Alcohol binges specifically increased NET formation in MASH livers in mice, and NETs were also increased in human livers with MASH plus alcohol use. We discovered that cell-free NETs are sensed via Nod-like receptor protein 3 (NLRP3). Furthermore, we show that cell-free NETs in vitro induce a profibrotic phenotype in hepatic stellate cells (HSCs) and proinflammatory monocytes. In vivo, neutrophil depletion using anti-Ly6G antibody or NET disruption with deoxyribonuclease treatment abrogated monocyte and HSC activation and ameliorated liver damage and fibrosis. In vivo, inhibition of NLRP3 using MCC950 or NLRP3 deficiency attenuated NET formation, liver injury and fibrosis in MASH plus alcohol diet-fed mice (graphical abstract).
Conclusion: Alcohol binges promote liver fibrosis via NET-induced activation of HSCs and monocytes in MASH. Our study highlights the potential of inhibition of NETs and/or NLRP3, as novel therapeutic strategies to combat the profibrotic effects of alcohol in MASH.
Keywords: ALCOHOLIC LIVER DISEASE; FIBROSIS; HEPATIC STELLATE CELL; MACROPHAGES.
© Author(s) (or their employer(s)) 2024. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GS reports being a paid consult for Durect Corporation, Cyta Therapeutics, Generon, Terra Firma, Quest Diagnostics, Pandion Therapeutics, Surrozen, Merck, Novartis, Pfizer, Lab Corp, Intercept and Takeda. She has stock options in Glympse Bio, Satellite Bio and Ventyx.
Figures





References
-
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019;70(1):151–71. - PubMed
-
- Boyle M, Masson S, Anstee QM. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J Hepatol 2018;68(2):251–67. - PubMed
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023. - PubMed
-
- Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, et al. Effects of Alcohol Consumption and Metabolic Syndrome on Mortality in Patients With Nonalcoholic and Alcohol-Related Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;17(8):1625–33 e1. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
