Comparison of Efficacy between Pemafibrate and Omega-3-Acid Ethyl Ester in the Liver: the PORTRAIT Study
- PMID: 38777770
- PMCID: PMC11537790
- DOI: 10.5551/jat.64896
Comparison of Efficacy between Pemafibrate and Omega-3-Acid Ethyl Ester in the Liver: the PORTRAIT Study
Abstract
Aim: No pharmacotherapeutic treatment has been established for metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH). This trial compared the effects of pemafibrate and omega-3-acid ethyl ester on hepatic function in patients with hypertriglyceridemia complicated by MASLD.
Methods: Patients with hypertriglyceridemia complicated by MASLD were enrolled, randomly assigned to the pemafibrate or omega-3-acid ethyl ester group, and followed for 24 weeks. The primary endpoint was the change in alanine aminotransferase (ALT) from baseline to week 24. The secondary endpoints included other hepatic enzymes, lipid profiles, and hepatic fibrosis biomarkers.
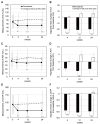
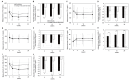
Results: A total of 80 patients were enrolled and randomized. The adjusted mean change in ALT from baseline to week 24 was significantly lower in the pemafibrate group (-19.7±5.9 U/L) than in the omega-3-acid ethyl ester group (6.8±5.5 U/L) (intergroup difference, -26.5 U/L; 95% confidence interval, -42.3 to -10.7 U/L; p=0.001). Pemafibrate significantly improved the levels of other hepatic enzymes (aspartate aminotransferase and gamma-glutamyl transpeptidase), lipid profiles (triglycerides, total cholesterol, high-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol), and hepatic fibrosis biomarkers (Mac-2 binding protein glycan isomer and Fibrosis-4 index). No cases of discontinuation due to adverse drug reactions were identified in either group, and there were no safety concerns.
Conclusions: Pemafibrate is recommended over omega-3-acid ethyl ester for lipid management and MASLD treatment in patients with hypertriglyceridemia complicated by MASLD. The study results may contribute to the development of future treatment strategies for patients with MASLD/MASH.
Keywords: Hepatic fibrosis; Hepatic function; Metabolic dysfunction-associated steatotic liver disease; Omega-3-acid ethyl ester; Pemafibrate.
Conflict of interest statement
Yoshio Sumida received a speaker’s bureau fee from Kowa Company, Ltd. Hidenori Toyoda received a speaker’s bureau fee from AbbVie GK, Abbott Japan LLC, Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Terumo Corporation, FUJIFILM Wako Pure Chemical Corporation, Eisai Co., Ltd., and Kowa Company, Ltd. Kiyoaki Ito received research funds from AbbVie GK, Gilead Sciences K.K., and Bristol Myers Squibb Company. Takeshi Osonoi received research funds from Kowa Company, Ltd. Masashi Yoneda received research funds from Kowa Company, Ltd., AbbVie GK, Bayer Yakuhin Ltd., Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., and Sumitomo Pharma Co. Ltd.; a consulting fee from Nitto Denko Corporation; and speaker’s bureau fees from Otsuka Pharmaceutical Co., Ltd. and Sumitomo Pharma Co. Ltd. Additionally, Masashi Yoneda holds leadership positions in the Japanese Society of Internal Medicine, Japanese Society of Gastroenterology, Japanese Society of Hepatology, and Japan Society of Neurovegetative Research. Satoshi Yasuda, Satoshi Kimoto, Kazumasa Sakamoto, and Yukiomi Nakade have no conflicts of interest to declare.
Figures
References
-
- Rinella ME, Lazarus JV, Ratziu V et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol, 2023; 79: 1542-1556 - PubMed
-
- Targher G, Kendrick J, Smits G, Chonchol M. Relationship between serum gamma-glutamyltransferase and chronic kidney disease in the United States adult population. Findings from the national health and nutrition examination survey 2001–2006. Nutr Metab Cardiovasc Dis, 2010; 20: 583-590 - PubMed
-
- Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care, 2007; 30: 2940-2944 - PubMed
-
- Akbar DH, Kawther AH. Nonalcoholic fatty liver disease in Saudi type 2 diabetic subjects attending a medical outpatient clinic: Prevalence and general characteristics. Diabetes Care, 2003; 26: 3351-3352 - PubMed
-
- Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol, 2004; 19: 854-858 - PubMed

