The β-arrestin1/endothelin axis bolsters ovarian fibroblast-dependent invadosome activity and cancer cell metastatic potential
- PMID: 38777849
- PMCID: PMC11111729
- DOI: 10.1038/s41419-024-06730-6
The β-arrestin1/endothelin axis bolsters ovarian fibroblast-dependent invadosome activity and cancer cell metastatic potential
Abstract
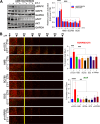
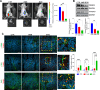
Recruitment of fibroblasts to tumors and their activation into cancer-associated fibroblasts (CAFs) is a strategy used by tumor cells to direct extracellular matrix (ECM) remodeling, invasion, and metastasis, highlighting the need to investigate the molecular mechanisms driving CAF function. Endothelin-1 (ET-1) regulates the communication between cancer and stroma and facilitates the progression of serous ovarian cancer (SOC). By binding to Endothelin A (ETA) and B (ETB) receptors, ET-1 enables the recruitment of β-arrestin1 (β-arr1) and the formation of signaling complexes that coordinate tumor progression. However, how ET-1 receptors might "educate" human ovarian fibroblasts (HOFs) to produce altered ECM and promote metastasis remains to be elucidated. This study identifies ET-1 as a pivotal factor in the activation of CAFs capable of proteolytic ECM remodeling and the generation of heterotypic spheroids containing cancer cells with a propensity to metastasize. An autocrine/paracrine ET-1/ETA/BR/β-arr1 loop enhances HOF proliferation, upregulates CAF marker expression, secretes pro-inflammatory cytokines, and increases collagen contractility, and cell motility. Furthermore, ET-1 facilitates ECM remodeling by promoting the lytic activity of invadosome and activation of integrin β1. In addition, ET-1 signaling supports the formation of heterotypic HOF/SOC spheroids with enhanced ability to migrate through the mesothelial monolayer, and invade, representing metastatic units. The blockade of ETA/BR or β-arr1 silencing prevents CAF activation, invadosome function, mesothelial clearance, and the invasive ability of heterotypic spheroids. In vivo, therapeutic inhibition of ETA/BR using bosentan (BOS) significantly reduces the metastatic potential of combined HOFs/SOC cells, associated with enhanced apoptotic effects on tumor cells and stromal components. These findings support a model in which ET-1/β-arr1 reinforces tumor/stroma interaction through CAF activation and fosters the survival and metastatic properties of SOC cells, which could be counteracted by ETA/BR antagonists.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
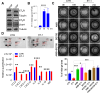
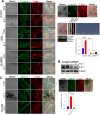
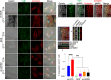
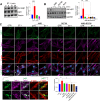

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

