One-year outcomes and safety assessment of faricimab in treatment-naïve patients with neovascular age-related macular degeneration in Japan
- PMID: 38778065
- PMCID: PMC11111667
- DOI: 10.1038/s41598-024-62559-1
One-year outcomes and safety assessment of faricimab in treatment-naïve patients with neovascular age-related macular degeneration in Japan
Abstract
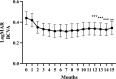
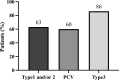
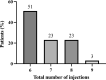
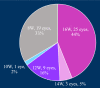
This multicentre retrospective study evaluated the 1-year outcomes and safety profile of faricimab in treatment-naïve patients with neovascular age-related macular degeneration (nAMD). Fifty-five patients (57 eyes) underwent loading therapy comprising three monthly faricimab injections. If dryness was achieved by the third month, subsequent treat-and-extend (TAE) follow-up continued at a minimum 8-week interval thereafter. If wet macula persisted at the third month, a fourth dose was administered, followed by the TAE regimen. After 1 year, improvements in visual acuity (0.44 ± 0.46 [baseline] to 0.34 ± 0.48; p < 0.01) and central foveal thickness (326 ± 149 [baseline] to 195 ± 82 μm; p < 0.0001) were significant. Dry macula, characterised by the absence of intraretinal or subretinal fluid, was achieved in 65% of cases. Treatment intervals varied, ranging from 8 to 16 weeks, with 44% of eyes extending to a 16-week interval, followed by 33% at 8 weeks, 16% at 12 weeks, 5% at 14 weeks, and 2% at 10 weeks. Notably, 50% of the polypoidal choroidal vasculopathy patients exhibited complete regression of polypoidal lesions between 12 and 15 months. Faricimab treatment in nAMD patients induced significant improvements in central vision and retinal morphology. Two cases of retinal pigment epithelial tears and one case of iritis were reported as ocular complications.
Keywords: Choroidal thickness; Faricimab; Neovascular age-related macular degeneration; Retinal morphology; Treat-and-extend regimen; Visual acuity.
© 2024. The Author(s).
Conflict of interest statement
R. Mukai, received lecturing and travel fees from Bayer Pharmaceuticals., Chugai Pharmaceutical., Novartis Pharma, Santen Pharmaceutical., and Senju Pharma.; K. Kataoka, received lecturing and travel fees from Santen Pharmaceutical, Bayer Pharmaceuticals, Senju Pharmaceutical, Japan Beringer Ingelheim, Otsuka Pharmaceutical, Canon Medtech, OMC Chugai Pharmaceutical, and Novartis Pharma.; K. Tanaka, received lecturing and travel fees from Santen Pharmaceutical, Alcon, Bayer Pharmaceuticals, Novartis Pharma, Senju Pharmaceutical, Nihon Tengan Kenkyusho, and Chugai Pharmaceutical.; Y. Miyara, received lecturing and travel fees from Senju Pharmaceutical.; I. Maruko, received lecturing and travel fees from Bayer Pharmaceuticals, Novartis Pharma, Japan Alcon, Santen, Senju Pharmaceutical, Topcon, Chugai Pharmaceutical, Canon, Nidek, and Nikon. He holds a patent.; M. Nakayama, None; Y. Watanabe, None; A. Yamamoto, received lecturing and travel fees from Novartis Pharma, Bayer Pharmaceuticals, and Chugai Pharmaceutical.; Y. Wakatsuki, None; H. Onoe, received lecturing and travel fees from Novartis Pharma, Senju Pharmaceutical, Chugai Pharmaceutical ; S. Wakugawa, received lecturing and travel fees from Senju Pharmaceutical and Novartis Pharma.; N. Terao, received lecturing and travel fees from Novartis Pharma, Bayer Pharmaceuticals, Santen Pharmaceutical, Abbott, Kowa, Topcon, Asahi Kasei Pharma, HOYA, and Chugai Pharmaceutical.; T. Hasegawa, received lecturing and travel fees from Bayer Pharmaceuticals, Novartis Pharma, Alcon Pharma, Santen, Kowa, Senju Pharmaceutical, R.E. Medical, Nikon Health Care Japan, JFC Sales Plan, Otsuka Pharmaceutical, and Japan Beringer Ingelheim.; M. Kawai, None; R. Maruko, received lecturing and travel fees from Kyowa Kirin.; K. Itagaki, received lecturing and travel fees from Novartis Pharma, Bayer, Santen Pharmaceutical, Senju Pharmaceutical, and Chugai Pharmaceutical.; J. Honjo, None; A.A. Okada, Personal fees from Alcon Pharma K.K., Allergan Japan, Apellis Pharmaceuticals Inc., Astellas Pharma Inc., Bayer Australia Ltd., Bayer Healthcare AG, Bayer Yakuhin Ltd., Biocon Biologics Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., Kowa Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., Otsuka Pharmaceutical Co. Ltd., Santen Pharmaceutical Co. Ltd., and Senju Pharmaceutical Co., Ltd. Departmental research grants from Alcon Pharma K.K., Bayer Yakuhin Ltd., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., and Santen Pharmaceuticals Co. Ltd.; R. Mori, received lecturing and travel fees from Santen Pharmaceutical, Bayer Pharmaceuticals, Japan Beringer Ingelheim, Kyowa Kirin, Novartis Pharma, Senju Pharmaceutical, and Chugai Pharmaceutical.; H. Koizumi, received grants from Novartis Pharma, Alcon Pharma, Japan Alcon, Bayer Pharmaceuticals, HOYA, Senju Pharmaceutical, Santen Pharmaceutical, AMO, Otsuka Pharmaceutical, Star Japan, First Medical, Pfizer, Kowa, and Nikon, received lecturing and travel fees from Novartis Pharma, Alcon Pharma, Bayer Pharmaceuticals, Senju Pharmaceutical, Santen Pharmaceutical class, Kowa, Japan Alcon, HOYA, AMO, Otsuka Pharmaceutical, Pfizer, Bausch + Lomb Japan, JFC Sales Plan, Canon, Nidek, Abbott, Tomey, Daiichi Sankyo, Chugai Pharmaceutical, and Sanofi, and received consultant fee from Novartis Pharma, Bayer Pharmaceuticals, Chugai Pharmaceutical, Allergan, Japan Beringer Ingelheim.; T. Iida, received grants from Nidek, Topcon, Santen, Novartis Pharma, Senju Pharmaceutical, Japan Alcon, HOYA, and AMO, consultant fee from Bayer Pharmaceuticals, Novartis Pharma, Chugai Pharmaceutical, Japan Beringer Ingelheim, and Janssen Pharma, and received lecturing and travel fees from Bayer Pharmaceuticals, Novartis Pharma, Japan Alcon, Santen, Senju Pharmaceutical, Topcon, Chugai Pharmaceutical, Canon, Nidek, Otsuka Pharmaceutical, Nikon, and Kyowa Kirin. He holds a patent.; T. Sekiryu, received grants from Novartis Pharma, Pfizer, Japan Alcon, Wakamoto Pharmaceutical, HOYA, Bayer Pharmaceuticals, Santen Pharmaceutical, Senju Pharmaceutical, AMO Japan, and Kowa, received lecturing and travel fees from Novartis Pharma, Chugai Pharmaceutical, Akura, Abbott, Alcon Pharma, Santen Pharmaceutical, and Senju Pharmaceutical, and received consultant fee from Allergan Japan.
Figures




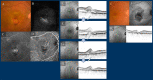

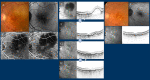
References
-
- Teutsch SM, Woodbury B, Welp A. Making Eye Health a Population Health Imperative: Vision for Tomorrow. The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press; 2016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

