Adhesive anti-fibrotic interfaces on diverse organs
- PMID: 38778109
- PMCID: PMC11168934
- DOI: 10.1038/s41586-024-07426-9
Adhesive anti-fibrotic interfaces on diverse organs
Erratum in
-
Author Correction: Adhesive anti-fibrotic interfaces on diverse organs.Nature. 2025 Jul;643(8074):E26. doi: 10.1038/s41586-025-09311-5. Nature. 2025. PMID: 40646128 Free PMC article. No abstract available.
Abstract
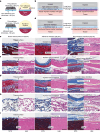
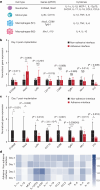
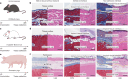
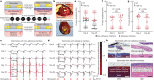
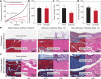
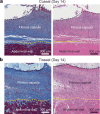
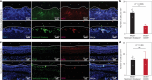
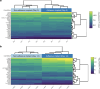
Implanted biomaterials and devices face compromised functionality and efficacy in the long term owing to foreign body reactions and subsequent formation of fibrous capsules at the implant-tissue interfaces1-4. Here we demonstrate that an adhesive implant-tissue interface can mitigate fibrous capsule formation in diverse animal models, including rats, mice, humanized mice and pigs, by reducing the level of infiltration of inflammatory cells into the adhesive implant-tissue interface compared to the non-adhesive implant-tissue interface. Histological analysis shows that the adhesive implant-tissue interface does not form observable fibrous capsules on diverse organs, including the abdominal wall, colon, stomach, lung and heart, over 12 weeks in vivo. In vitro protein adsorption, multiplex Luminex assays, quantitative PCR, immunofluorescence analysis and RNA sequencing are additionally carried out to validate the hypothesis. We further demonstrate long-term bidirectional electrical communication enabled by implantable electrodes with an adhesive interface over 12 weeks in a rat model in vivo. These findings may offer a promising strategy for long-term anti-fibrotic implant-tissue interfaces.
© 2024. The Author(s).
Conflict of interest statement
H.Y. and X.Z. have a financial interest in SanaHeal. X.Z. has a financial interest in SonoLogi. J.W., J.D., H.Y. and X.Z. are inventors of a patent application that covers the adhesive anti-fibrotic interfaces. The other authors declare no competing interests.
Figures








References
-
- Anderson, J. M. Biological responses to materials. Annu. Rev. Mater. Res.31, 81–110 (2001).
-
- Wick, G. et al. The immunology of fibrosis. Annu. Rev. Immunol.31, 107–135 (2013). - PubMed
-
- Chandorkar, Y. & Basu, B. The foreign body response demystified. ACS Biomater. Sci. Eng.5, 19–44 (2018). - PubMed
-
- Harding, J. L. & Reynolds, M. M. Combating medical device fouling. Trends Biotechnol.32, 140–146 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

