Simple Behavioral Analysis (SimBA) as a platform for explainable machine learning in behavioral neuroscience
- PMID: 38778146
- PMCID: PMC11268425
- DOI: 10.1038/s41593-024-01649-9
Simple Behavioral Analysis (SimBA) as a platform for explainable machine learning in behavioral neuroscience
Abstract
The study of complex behaviors is often challenging when using manual annotation due to the absence of quantifiable behavioral definitions and the subjective nature of behavioral annotation. Integration of supervised machine learning approaches mitigates some of these issues through the inclusion of accessible and explainable model interpretation. To decrease barriers to access, and with an emphasis on accessible model explainability, we developed the open-source Simple Behavioral Analysis (SimBA) platform for behavioral neuroscientists. SimBA introduces several machine learning interpretability tools, including SHapley Additive exPlanation (SHAP) scores, that aid in creating explainable and transparent behavioral classifiers. Here we show how the addition of explainability metrics allows for quantifiable comparisons of aggressive social behavior across research groups and species, reconceptualizing behavior as a sharable reagent and providing an open-source framework. We provide an open-source, graphical user interface (GUI)-driven, well-documented package to facilitate the movement toward improved automation and sharing of behavioral classification tools across laboratories.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures


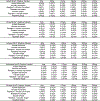
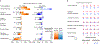



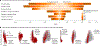


References
References,,,–
Methods only references:
MeSH terms
Grants and funding
- K08 MH123791/MH/NIMH NIH HHS/United States
- 4R00DA045662-02/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- R01 DA059374/DA/NIDA NIH HHS/United States
- R00 DA045662/DA/NIDA NIH HHS/United States
- R35GM146751/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- F31 AA025827/AA/NIAAA NIH HHS/United States
- K08MH123791/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R35 GM146751/GM/NIGMS NIH HHS/United States
- 1F31MH125587-01/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- F31AA025827/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- DA048736/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- F31 MH125587/MH/NIMH NIH HHS/United States
- 27082/Brain and Behavior Research Foundation (Brain & Behavior Research Foundation)
- T32 HL007028/HL/NHLBI NIH HHS/United States
- F32MH125634/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- P30 DA048736/DA/NIDA NIH HHS/United States
- K99 MH133869/MH/NIMH NIH HHS/United States
- P30DA048736/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- F32 MH125634/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

