Transcranial volumetric imaging using a conformal ultrasound patch
- PMID: 38778234
- PMCID: PMC11875229
- DOI: 10.1038/s41586-024-07381-5
Transcranial volumetric imaging using a conformal ultrasound patch
Abstract
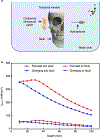
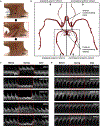
Accurate and continuous monitoring of cerebral blood flow is valuable for clinical neurocritical care and fundamental neurovascular research. Transcranial Doppler (TCD) ultrasonography is a widely used non-invasive method for evaluating cerebral blood flow1, but the conventional rigid design severely limits the measurement accuracy of the complex three-dimensional (3D) vascular networks and the practicality for prolonged recording2. Here we report a conformal ultrasound patch for hands-free volumetric imaging and continuous monitoring of cerebral blood flow. The 2 MHz ultrasound waves reduce the attenuation and phase aberration caused by the skull, and the copper mesh shielding layer provides conformal contact to the skin while improving the signal-to-noise ratio by 5 dB. Ultrafast ultrasound imaging based on diverging waves can accurately render the circle of Willis in 3D and minimize human errors during examinations. Focused ultrasound waves allow the recording of blood flow spectra at selected locations continuously. The high accuracy of the conformal ultrasound patch was confirmed in comparison with a conventional TCD probe on 36 participants, showing a mean difference and standard deviation of difference as -1.51 ± 4.34 cm s-1, -0.84 ± 3.06 cm s-1 and -0.50 ± 2.55 cm s-1 for peak systolic velocity, mean flow velocity, and end diastolic velocity, respectively. The measurement success rate was 70.6%, compared with 75.3% for a conventional TCD probe. Furthermore, we demonstrate continuous blood flow spectra during different interventions and identify cascades of intracranial B waves during drowsiness within 4 h of recording.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures








References
-
- Aaslid R (ed.) Transcranial Doppler Sonography (Springer Science+Business Media, 2012).
-
- Alexandrov AV et al. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J. Neuroimaging 22, 215–224 (2012). - PubMed
-
- Lohmann H, Ringelstein EB & Knecht S in Handbook on Neurovascular Ultrasound Vol. 21 (ed. Baumgartner RW) 251–260 (Karger, 2006).
-
- Gambhir SS Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 (2002). - PubMed
-
- Withers PJ et al. X-ray computed tomography. Nat. Rev. Methods Primers 1, 18 (2021).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

