In vitro induction of tetraploids and their phenotypic and transcriptome analysis in Glehnia littoralis
- PMID: 38778255
- PMCID: PMC11110393
- DOI: 10.1186/s12870-024-05154-w
In vitro induction of tetraploids and their phenotypic and transcriptome analysis in Glehnia littoralis
Abstract
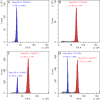
Background: Glehnia littoralis is a medicinal and edible plant species having commercial value and has several hundred years of cultivation history. Polyploid breeding is one of the most important and fastest ways to generate novel varieties. To obtain tetraploids of G. littoralis in vitro, colchicine treatment was given to the seeds and then were screened based on morphology, flow cytometry, and root tip pressing assays. Furthermore, transcriptome analysis was performed to identity the differentially expressed genes associated with phenotypic changes in tetraploid G. littoralis.
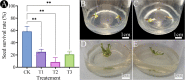
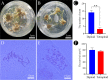
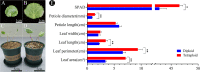
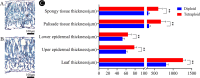
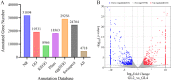
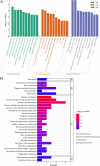
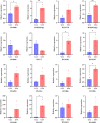
Results: The results showed that 0.05% (w/v) colchicine treatment for 48 h was effective in inducing tetraploids in G. littoralis. The tetraploid G. littoralis (2n = 4x = 44) was superior in leaf area, leaf thickness, petiole diameter, SPAD value (Chl SPAD), stomatal size, epidermal tissues thickness, palisade tissues thickness, and spongy tissues thickness to the diploid ones, while the stomatal density of tetraploids was significantly lower. Transcriptome sequencing revealed, a total of 1336 differentially expressed genes (DEGs) between tetraploids and diploids. Chromosome doubling may lead to DNA content change and gene dosage effect, which directly affects changes in quantitative traits, with changes such as increased chlorophyll content, larger stomata and thicker tissue of leaves. Several up-regulated DEGs were found related to growth and development in tetraploid G. littoralis such as CKI, PPDK, hisD and MDP1. KEGG pathway enrichment analyses showed that most of DEGs were enriched in metabolic pathways.
Conclusions: This is the first report of the successful induction of tetraploids in G. littoralis. The information presented in this study facilitate breeding programs and molecular breeding of G. littoralis varieties.
Keywords: Glehnia littoralis; Leaf; Phenotype; Tetraploid; Transcriptome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Comparison of leaf transcriptomes of cassava "Xinxuan 048" diploid and autotetraploid plants.Genes Genomics. 2018 Sep;40(9):927-935. doi: 10.1007/s13258-018-0692-2. Epub 2018 May 15. Genes Genomics. 2018. PMID: 30155710
-
Character changes and Transcriptomic analysis of a cassava sexual Tetraploid.BMC Plant Biol. 2021 Apr 19;21(1):188. doi: 10.1186/s12870-021-02963-1. BMC Plant Biol. 2021. PMID: 33874893 Free PMC article.
-
In vitro induction of tetraploidy and its effects on phenotypic variations in Populus hopeiensis.BMC Plant Biol. 2023 Nov 13;23(1):557. doi: 10.1186/s12870-023-04578-0. BMC Plant Biol. 2023. PMID: 37957587 Free PMC article.
-
Induction of tetraploids in Paper Mulberry (Broussonetia papyrifera (L.) L'Hér. ex Vent.) by colchicine.BMC Plant Biol. 2023 Nov 17;23(1):574. doi: 10.1186/s12870-023-04487-2. BMC Plant Biol. 2023. PMID: 37978431 Free PMC article.
-
The association analysis of DNA methylation and transcriptomics identified BpCYCD3;2 as a participant in influencing cell division in autotetraploid birch (Betula pendula) leaves.Plant Sci. 2024 Jul;344:112099. doi: 10.1016/j.plantsci.2024.112099. Epub 2024 Apr 17. Plant Sci. 2024. PMID: 38640971
Cited by
-
Efficient induction of tetraploids via adventitious bud regeneration and subsequent phenotypic variation in Acacia melanoxylon.Plant Methods. 2025 Aug 22;21(1):115. doi: 10.1186/s13007-025-01426-0. Plant Methods. 2025. PMID: 40847368 Free PMC article.
-
Comparison of the morphological and physiological characteristics of diploid and tetraploid Luculia pinceana Hook.BMC Plant Biol. 2025 Apr 25;25(1):536. doi: 10.1186/s12870-025-06568-w. BMC Plant Biol. 2025. PMID: 40281416 Free PMC article.
References
-
- Diem LT, Phong TH, Tung HT, Khai HD, Anh TTL, Mai NTN, et al. Tetraploid induction through somatic embryogenesis in Panax vietnamensis ha et grushv. By colchicine treatment. Sci Hortic. 2022;303:111254. doi: 10.1016/j.scienta.2022.111254. - DOI
-
- Iannicelli J, Guariniello J, Tossi VE, Regalado JJ, Di Ciaccio L, Van Baren CM, et al. The polyploid effect in the breeding of aromatic and medicinal species. Sci Hortic. 2020;260:108854. doi: 10.1016/j.scienta.2019.108854. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

