The use of probiotics and prebiotics in decolonizing pathogenic bacteria from the gut; a systematic review and meta-analysis of clinical outcomes
- PMID: 38778521
- PMCID: PMC11123511
- DOI: 10.1080/19490976.2024.2356279
The use of probiotics and prebiotics in decolonizing pathogenic bacteria from the gut; a systematic review and meta-analysis of clinical outcomes
Abstract
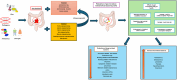
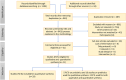
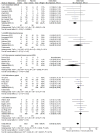
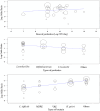
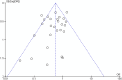
Repeated exposure to antibiotics and changes in the diet and environment shift the gut microbial diversity and composition, making the host susceptible to pathogenic infection. The emergence and ongoing spread of AMR pathogens is a challenging public health issue. Recent evidence showed that probiotics and prebiotics may play a role in decolonizing drug-resistant pathogens by enhancing the colonization resistance in the gut. This review aims to analyze available evidence from human-controlled trials to determine the effect size of probiotic interventions in decolonizing AMR pathogenic bacteria from the gut. We further studied the effects of prebiotics in human and animal studies. PubMed, Embase, Web of Science, Scopus, and CINAHL were used to collect articles. The random-effects model meta-analysis was used to pool the data. GRADE Pro and Cochrane collaboration tools were used to assess the bias and quality of evidence. Out of 1395 citations, 29 RCTs were eligible, involving 2871 subjects who underwent either probiotics or placebo treatment to decolonize AMR pathogens. The persistence of pathogenic bacteria after treatment was 22%(probiotics) and 30.8%(placebo). The pooled odds ratio was 0.59(95% CI:0.43-0.81), favoring probiotics with moderate certainty (p = 0.0001) and low heterogeneity (I2 = 49.2%, p = 0.0001). The funnel plot showed no asymmetry in the study distribution (Kendall'sTau = -1.06, p = 0.445). In subgroup, C. difficile showed the highest decolonization (82.4%) in probiotics group. Lactobacillus-based probiotics and Saccharomyces boulardii decolonize 71% and 77% of pathogens effectively. The types of probiotics (p < 0.018) and pathogens (p < 0.02) significantly moderate the outcome of decolonization, whereas the dosages and regions of the studies were insignificant (p < 0.05). Prebiotics reduced the pathogens from 30% to 80% of initial challenges. Moderate certainty of evidence suggests that probiotics and prebiotics may decolonize pathogens through modulation of gut diversity. However, more clinical outcomes are required on particular strains to confirm the decolonization of the pathogens. Protocol registration: PROSPERO (ID = CRD42021276045).
Keywords: Probiotics; alpha diversity; decolonization; gut microbiota; prebiotics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
