The prognostic marker KRT81 is involved in suppressing CD8 + T cells and predicts immunotherapy response for triple-negative breast cancer
- PMID: 38778753
- PMCID: PMC11123506
- DOI: 10.1080/15384047.2024.2355705
The prognostic marker KRT81 is involved in suppressing CD8 + T cells and predicts immunotherapy response for triple-negative breast cancer
Abstract
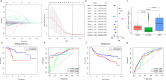
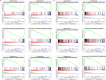
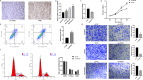
Triple-negative breast Cancer (TNBC) is an aggressive subtype lacking estrogen, progesterone, and HER2 receptors. Known for limited targeted therapies, it poses challenges and requires personalized treatment strategies. Differential analysis revealed a significant decrease in keratin 81 (KRT81) expression in non-TNBC samples and an increase in TNBC samples, lower KRT81 expression correlated with better TNBC patient outcomes. It emerged as an independent predictive factor for TNBC, with associations found between its expression and clinically relevant features. We further developed a nomogram for survival probability assessment based on Cox regression results, demonstrating its accuracy through calibration curves. Gene annotation analysis indicated that KRT81 is involved in immune-related pathways and tumor cell adhesion. KRT81 is associated with immune cell infiltration of Follicular helper T cells (Tfh) and CD8 + T cells, suggesting its potential impact on the immunological microenvironment. The study delved into KRT81's predictive value for immunotherapy responses, high expression of KRT81 was associated with greater potential for immune evasion. Single-cell RNA sequencing analysis pinpointed KRT81 expression within a specific malignant subtype which was a risk factor for TNBC. Furthermore, KRT81 promoted TNBC cell proliferation, migration, invasion, and adhesion was confirmed by gene knockout or overexpression assay. Co-culture experiments further indicated KRT81's potential role in inhibiting CD8 + T cells, and correlation analysis implied KRT81 was highly correlated with immune checkpoint CD276, providing insights into its involvement in the immune microenvironment via CD276. In conclusion, this comprehensive study positions KRT81 as a promising prognostic marker for predicting tumor progression and immunotherapy responses in TNBC.
Keywords: CD8 + T cells; Triple-negative breast cancer; immune microenvironment; immunotherapy response; keratin 81; prognostic gene.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





Similar articles
-
Construction of a stromal cell-related prognostic signature based on a 101-combination machine learning framework for predicting prognosis and immunotherapy response in triple-negative breast cancer.Front Immunol. 2025 May 14;16:1544348. doi: 10.3389/fimmu.2025.1544348. eCollection 2025. Front Immunol. 2025. PMID: 40438115 Free PMC article.
-
CD8+ T cell‑related KCTD5 contributes to malignant progression and unfavorable clinical outcome of patients with triple‑negative breast cancer.Mol Med Rep. 2024 Sep;30(3):166. doi: 10.3892/mmr.2024.13290. Epub 2024 Jul 19. Mol Med Rep. 2024. PMID: 39027992 Free PMC article.
-
ALG3 predicts poor prognosis and increases resistance to anti-PD-1 therapy through modulating PD-L1 N-link glycosylation in TNBC.Int Immunopharmacol. 2024 Oct 25;140:112875. doi: 10.1016/j.intimp.2024.112875. Epub 2024 Aug 9. Int Immunopharmacol. 2024. PMID: 39116492
-
CD8+ T-cell exhaustion: Impediment to triple-negative breast cancer (TNBC) immunotherapy.Biochim Biophys Acta Rev Cancer. 2024 Nov;1879(6):189193. doi: 10.1016/j.bbcan.2024.189193. Epub 2024 Oct 15. Biochim Biophys Acta Rev Cancer. 2024. PMID: 39413858 Review.
-
Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects.Drug Resist Updat. 2017 May;32:1-15. doi: 10.1016/j.drup.2017.07.002. Epub 2017 Aug 19. Drug Resist Updat. 2017. PMID: 29145974 Review.
Cited by
-
UNC93B1: a novel immune-related prognostic biomarker in breast cancer.Discov Oncol. 2025 Jul 17;16(1):1352. doi: 10.1007/s12672-025-03124-8. Discov Oncol. 2025. PMID: 40673974 Free PMC article.
-
CD4+ T cells in antitumor immunity.Trends Cancer. 2024 Oct;10(10):969-985. doi: 10.1016/j.trecan.2024.07.009. Epub 2024 Sep 5. Trends Cancer. 2024. PMID: 39242276 Review.
-
Single-cell transcriptome profiling reveals dynamic cell populations and immune infiltration in cerebral cavernous malformation.Front Immunol. 2025 May 30;16:1592343. doi: 10.3389/fimmu.2025.1592343. eCollection 2025. Front Immunol. 2025. PMID: 40519934 Free PMC article.
-
Evaluation of prognostic risk factors of triple-negative breast cancer with 18F-FDG PET/CT parameters, clinical pathological features and biochemical indicators.Front Cell Dev Biol. 2024 Sep 4;12:1421981. doi: 10.3389/fcell.2024.1421981. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39296933 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
