Potential consumer response to the healthy symbol proposed by the U.S. food and Drug Administration
- PMID: 38778947
- PMCID: PMC11109763
- DOI: 10.1016/j.heliyon.2024.e30863
Potential consumer response to the healthy symbol proposed by the U.S. food and Drug Administration
Abstract
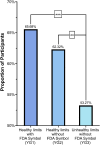
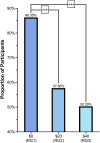
The U.S. Food and Drug Administration (FDA) has proposed updates to the definition of "healthy," including distinctions between types of sugar and fats and limits on added sugar, saturated fat, and sodium. To communicate the updated standards, the FDA is developing a Healthy symbol to display on food packages, which could reduce knowledge gaps by assisting U.S. consumers in meeting recommended nutritional guidelines. This study aimed to explore the potential for the label to increase consumers' ability to correctly identify a food product that met the FDA's criteria for a healthy symbol. To complete the study objective, 1018 adults were recruited to represent the U.S. population regarding gender, age, income, and geographic region, and a randomized group experiment was used to determine the potential communication value of an FDA Healthy symbol. Respondents were randomized to a group shown either a healthy yogurt with the FDA symbol, a healthy yogurt without the symbol, or an unhealthy yogurt. Respondents were then asked whether they considered the yogurt shown to be healthy, a question examining the desired criteria for the Healthy symbol, willingness to accept various costs to implement the symbol, and questions to measure objective dietary knowledge. Adding the symbol to yogurt that already met the healthy criteria only yielded about a 4 percentage point increase in the proportion of respondents identifying it as healthy. However, 53 % of participants still identified a yogurt too high in added sugars as healthy. For the desired label criteria, 64 % of respondents selected limits on added sugars, 57 % selected limits on sodium, and 54 % selected limits on saturated fats, which all align with the proposed updates to the definition of healthy. Over half of the participants supported the implementation of the label, even at a cost of $40 annually, and 86 % supported implementation at no cost.
Keywords: Consumer behavior; Dietary intake; Food labeling; Nutrition.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- U.S. Food and Drug Administration (USFDA) Food labeling: Nutrient content claims; Definition of the term "healthy". Fed. Regist. 2022;87(188):59168–59202. https://www.federalregister.gov/documents/2022/09/29/2022-20975/food-lab... Retrieved January 17, 2023, from. - PubMed
-
- U.S. Food and Drug Administration (USFDA) 1994. Food Labeling: Nutrient Content Claims; Definition of Term Healthy.https://archives.federalregister.gov/issue_slice/1994/5/10/24216-24254.p... Retrieved June 6, 2023, from. - PubMed
-
- U.S. Department of Agriculture (USDA) and U.S. Department of Health and Human Services (USHHS). Dietary Guidelines for Americans, 2020-2025. ninth ed. December 2020. Available at: DietaryGuidelines.gov)
-
- U.S. Food and Drug Administration (USFDA) Food labeling; Revision of the nutrition and Supplement facts labels; Technical Amendments. 2018. https://www.federalregister.gov/documents/2018/12/21/2018-27431/food-lab... Retrieved June 6, 2023, from. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

