Chronic wasting disease alters the movement behavior and habitat use of mule deer during clinical stages of infection
- PMID: 38779534
- PMCID: PMC11108800
- DOI: 10.1002/ece3.11418
Chronic wasting disease alters the movement behavior and habitat use of mule deer during clinical stages of infection
Abstract
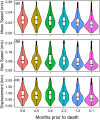
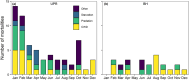
Integrating host movement and pathogen data is a central issue in wildlife disease ecology that will allow for a better understanding of disease transmission. We examined how adult female mule deer (Odocoileus hemionus) responded behaviorally to infection with chronic wasting disease (CWD). We compared movement and habitat use of CWD-infected deer (n = 18) to those that succumbed to starvation (and were CWD-negative by ELISA and IHC; n = 8) and others in which CWD was not detected (n = 111, including animals that survived the duration of the study) using GPS collar data from two distinct populations collared in central Wyoming, USA during 2018-2022. CWD and predation were the leading causes of mortality during our study (32/91 deaths attributed to CWD and 27/91 deaths attributed to predation). Deer infected with CWD moved slower and used lower elevation areas closer to rivers in the months preceding death compared with uninfected deer that did not succumb to starvation. Although CWD-infected deer and those that died of starvation moved at similar speeds during the final months of life, CWD-infected deer used areas closer to streams with less herbaceous biomass than starved deer. These behavioral differences may allow for the development of predictive models of disease status from movement data, which will be useful to supplement field and laboratory diagnostics or when mortalities cannot be quickly retrieved to assess cause-specific mortality. Furthermore, identifying individuals who are sick before predation events could help to assess the extent to which disease mortality is compensatory with predation. Finally, infected animals began to slow down around 4 months prior to death from CWD. Our approach for detecting the timing of infection-induced shifts in movement behavior may be useful in application to other disease systems to better understand the response of wildlife to infectious disease.
Keywords: Bayesian inference; Cervidae; behavioral change; cause‐specific mortality; chronic wasting disease; host–pathogen dynamics; space use; transmissible spongiform encephalopathy.
© 2024 The Author(s). Ecology and Evolution published by John Wiley & Sons Ltd. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Albery, G. F. , Kirkpatrick, L. , Firth, J. A. , & Bansal, S. (2021). Unifying spatial and social network analysis in disease ecology. Journal of Animal Ecology, 90(1), 45–61. - PubMed
-
- Barrile, G. M. , Chalfoun, A. D. , & Walters, A. W. (2021). Infection status as the basis for habitat choices in a wild amphibian. The American Naturalist, 197(1), 128–137. - PubMed
-
- Bergman, E. J. , Doherty, P. F., Jr. , White, G. C. , & Holland, A. A. (2015). Density dependence in mule deer: A review of evidence. Wildlife Biology, 21(1), 18–29.
Grants and funding
LinkOut - more resources
Full Text Sources

