Piezoelectrically-activated antibacterial catheter for prevention of urinary tract infections in an on-demand manner
- PMID: 38779557
- PMCID: PMC11109010
- DOI: 10.1016/j.mtbio.2024.101089
Piezoelectrically-activated antibacterial catheter for prevention of urinary tract infections in an on-demand manner
Abstract
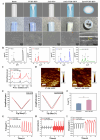
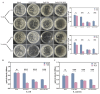
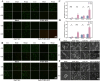
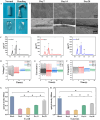
Catheter-associated urinary tract infection (CAUTI) is a common clinical problem, especially during long-term catheterization, causing additional pain to patients. The development of novel antimicrobial coatings is needed to prolong the service life of catheters and reduce the incidence of CAUTIs. Herein, we designed an antimicrobial catheter coated with a piezoelectric zinc oxide nanoparticles (ZnO NPs)-incorporated polyvinylidene difluoride-hexafluoropropylene (ZnO-PVDF-HFP) membrane. ZnO-PVDF-HFP could be stably coated onto silicone catheters simply by a one-step solution film-forming method, very convenient for industrial production. In vitro, it was demonstrated that ZnO-PVDF-HFP coating could significantly inhibit bacterial growth and the formation of bacterial biofilm under ultrasound-mediated mechanical stimulation even after 4 weeks. Importantly, the on and off of antimicrobial activity as well as the strenth of antibacterial property could be controlled in an adaptive manner via ultrasound. In a rabbit model, the ZnO-PVDF-HFP-coated catheter significantly reduced the incidence CAUTIs compared with clinically-commonly used catheters under assistance of ultrasonication, and no side effect was detected. Collectively, the study provided a novel antibacterial catheter to prevent the occurrence of CAUTIs, whose antibacterial activity could be controlled in on-demand manner, adaptive to infection situation and promising in clinical application.
Keywords: Anti-infection; Nano-zinc oxide; Piezoelectricity; ROS; Uinary catheter.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Sonochemically engineered nano-enabled zinc oxide/amylase coatings prevent the occurrence of catheter-associated urinary tract infections.Mater Sci Eng C Mater Biol Appl. 2021 Dec;131:112518. doi: 10.1016/j.msec.2021.112518. Epub 2021 Oct 26. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34857297
-
ZnO@Carbon Dot Nanoparticles Stimulating the Antibacterial Activity of Polyvinylidene Fluoride-Hexafluoropropylene with a Higher Electroactive Phase for Multifunctional Devices.ACS Appl Mater Interfaces. 2023 Feb 8;15(5):6735-6746. doi: 10.1021/acsami.2c18859. Epub 2023 Jan 25. ACS Appl Mater Interfaces. 2023. PMID: 36696096
-
Developing a Urinary Catheter with Anti-Biofilm Coated Surface Using Phyto-Assisted Synthesis of Zinc Oxide Nanoparticles.Infect Drug Resist. 2025 Apr 14;18:1881-1893. doi: 10.2147/IDR.S509957. eCollection 2025. Infect Drug Resist. 2025. PMID: 40255461 Free PMC article.
-
Catheter-associated urinary tract infections: new aspects of novel urinary catheters.Int J Antimicrob Agents. 2006 Dec;28(6):485-90. doi: 10.1016/j.ijantimicag.2006.08.020. Epub 2006 Oct 12. Int J Antimicrob Agents. 2006. PMID: 17045784 Review.
-
Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: A review on recent updates.J Infect Public Health. 2019 Nov-Dec;12(6):760-766. doi: 10.1016/j.jiph.2019.09.009. Epub 2019 Oct 16. J Infect Public Health. 2019. PMID: 31628048 Review.
Cited by
-
Covalent Grafting of Quaternary Ammonium Salt-Containing Polyurethane onto Silicone Substrates to Enhance Bacterial Contact-Killing Ability.Polymers (Basel). 2024 Dec 25;17(1):17. doi: 10.3390/polym17010017. Polymers (Basel). 2024. PMID: 39795421 Free PMC article.
-
Recent Progress in Antibacterial Surfaces for Implant Catheters.BME Front. 2025 Feb 13;6:0063. doi: 10.34133/bmef.0063. eCollection 2025. BME Front. 2025. PMID: 39949607 Free PMC article.
-
A chitosan/acellular matrix-based neural graft carrying mesenchymal stem cells to promote peripheral nerve repair.Stem Cell Res Ther. 2024 Dec 31;15(1):503. doi: 10.1186/s13287-024-04093-5. Stem Cell Res Ther. 2024. PMID: 39736729 Free PMC article.
-
Porphyrin-based porous organic polymer for the NIR-enhanced delivery of bupivacaine towards pain management in bacterial infection therapy.RSC Adv. 2025 Feb 3;15(5):3353-3364. doi: 10.1039/d4ra06433j. eCollection 2025 Jan 29. RSC Adv. 2025. PMID: 39902106 Free PMC article.
References
LinkOut - more resources
Full Text Sources

