Large-scale production of the malaria vector biocontrol agent Romanomermis iyengari (Nematoda: Mermithidae) in Benin, West Africa
- PMID: 38779621
- PMCID: PMC11107864
- DOI: 10.5281/zenodo.10869973
Large-scale production of the malaria vector biocontrol agent Romanomermis iyengari (Nematoda: Mermithidae) in Benin, West Africa
Abstract
Background: The mermithid nematode Romanomermis iyengari is one of several natural control alternatives to synthetic pesticides for mosquito suppression. The commonly used mass rearing procedure of R. iyengari involves the use of coarse sand as a substrate for nematode maturation and oviposition. The coarse sand technique gives excellent nematode productivity in North America. However, under West African climatic conditions, this technique generates relatively lesser amounts of infectious worms. We evaluated coconut coir fibres as a replacement for coarse sand to improve yields in large-scale production of R. iyengari in Benin, West Africa.
Materials and methods: Culex quinquefasciatus was the host for the nematodes, and mosquitoes were blood-fed on chickens. Four days after blood feeding, egg rafts were collected and transferred into trays, each containing 2 l of water. The mosquito larvae were fed with fish food. When the mosquito larvae reached the second instar, preparasites (J2) were added (3 J2/larva) to the incubation trays. Eight days after infection, post-parasitic juveniles were separated from the water containing dead mosquito larvae and other debris using sieves and needles; 2 g of them were deposited in containers with coarse sand or coconut coir fibres and water. Three hours later, the water was drained, the jars covered and stored for eight weeks, after which J2 abundance was determined, using a total of 320 containers for each substrate. The abundance of J2 preparasites was also assessed 3-5 months after storage to determine the impact of long-term storage on the J2 yield.
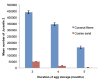
Results: After 2 months storage, 2 g of post-parasites (~457 females and 583 males) yielded an average of 559,300±6094 J2 and 155,818±4427 J2 per container for coconut fibres and for coarse sand, respectively. During long-term storage, yields of J2 on coconut fibres substrate slowly decreased from 442,180±9322 J2 (3 months storage) to 163,632±12,416 J2 per container (5 months storage). On coarse sand substrate, the yield was relatively low and decreased from 49,812±1200 J2 at 3 months storage to 3046±229 J2 at 5 months storage.
Conclusion: Under West African climatic conditions, coconut coir fibres gave significantly higher preparasitic nematode yields than the coarse sand technique.
Copyright © 2015; Alavo et al.
Conflict of interest statement
Competing interests: No competing interests declared.
Figures




Similar articles
-
Efficacy of the mermithid nematode, Romanomermis iyengari, for the biocontrol of Anopheles gambiae, the major malaria vector in sub-Saharan Africa.Parasit Vectors. 2019 May 22;12(1):253. doi: 10.1186/s13071-019-3508-6. Parasit Vectors. 2019. PMID: 31118105 Free PMC article.
-
Biocontrol of Culex quinquefasciatus using the insect parasitic nematode, Romanomermis iyengari (Nematoda: Mermithidae).Trop Biomed. 2019 Dec 1;36(4):1003-1013. Trop Biomed. 2019. PMID: 33597470
-
Susceptibility of ten species of mosquito larvae to the parasitic nematode Romanomermis iyengari and its development.Med Vet Entomol. 2000 Dec;14(4):426-9. doi: 10.1046/j.1365-2915.2000.00263.x. Med Vet Entomol. 2000. PMID: 11129707
-
Host Penetration and Emergence Patterns of the Mosquito-Parasitic Mermithids Romanomermis iyengari and Strelkovimermis spiculatus (Nematoda: Mermithidae).J Nematol. 2013 Mar;45(1):30-8. J Nematol. 2013. PMID: 23589657 Free PMC article.
-
An improved method of mass culturing of Romanomermis iyengari, a mermithid nematode parasite of mosquito larvae.Indian J Med Res. 1990 Jul;91:298-302. Indian J Med Res. 1990. PMID: 2228062
Cited by
-
Superparasitism and Population Regulation of the Mosquito-Parasitic Mermithid Nematodes Romanomermis iyengari and Strelkovimermis spiculatus.J Nematol. 2017 Sep;49(3):316-320. J Nematol. 2017. PMID: 29062155 Free PMC article.
-
Efficacy of the mermithid nematode, Romanomermis iyengari, for the biocontrol of Anopheles gambiae, the major malaria vector in sub-Saharan Africa.Parasit Vectors. 2019 May 22;12(1):253. doi: 10.1186/s13071-019-3508-6. Parasit Vectors. 2019. PMID: 31118105 Free PMC article.
References
-
- Djogbénou L, Pasteur N, Akogbéto M, Weill M et al. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med. Vet. Entomol. 2011;25:256–267. - PubMed
-
- Gajanana A, Kazmin SJ, Bheema Rao US, Suguna SG et al. Studies on a nematode parasite (Romanomermis sp.: Mermithidae) of mosquito larvae isolated in Pondicherry. Indian J. Med. Res. 1978;68:242–247. - PubMed
-
- Iyengar MOT: Parasitic nematodes of Anopheles in Bengal. Far Eastern Association for Tropical Medicine, Transactions 7th Cong. British India, 1927;3:128–135.
-
- Chandrahas RK, Rajagopalan PK: Mosquito breeding and the natural parasitism of larvae by a fungus Coelomomyces and mermithid nematode Romanomermis in paddy fields in Pondicherry. Indian J. Med. Res. 1979;69:63–70. - PubMed
-
- Platzer EG: Mermithid nematodes. J. Am. Mosq. Control Assoc. 2007;23:58–64. - PubMed
LinkOut - more resources
Full Text Sources
