Interstitial macrophage phenotypes in Schistosoma-induced pulmonary hypertension
- PMID: 38779688
- PMCID: PMC11109442
- DOI: 10.3389/fimmu.2024.1372957
Interstitial macrophage phenotypes in Schistosoma-induced pulmonary hypertension
Abstract
Background: Schistosomiasis is a common cause of pulmonary hypertension (PH) worldwide. Type 2 inflammation contributes to the development of Schistosoma-induced PH. Specifically, interstitial macrophages (IMs) derived from monocytes play a pivotal role by producing thrombospondin-1 (TSP-1), which in turn activates TGF-β, thereby driving the pathology of PH. Resident and recruited IM subpopulations have recently been identified. We hypothesized that in Schistosoma-PH, one IM subpopulation expresses monocyte recruitment factors, whereas recruited monocytes become a separate IM subpopulation that expresses TSP-1.
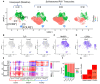
Methods: Mice were intraperitoneally sensitized and then intravenously challenged with S. mansoni eggs. Flow cytometry on lungs and blood was performed on wildtype and reporter mice to identify IM subpopulations and protein expression. Single-cell RNA sequencing (scRNAseq) was performed on flow-sorted IMs from unexposed and at day 1, 3 and 7 following Schistosoma exposure to complement flow cytometry based IM characterization and identify gene expression.
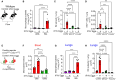
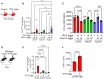
Results: Flow cytometry and scRNAseq both identified 3 IM subpopulations, characterized by CCR2, MHCII, and FOLR2 expression. Following Schistosoma exposure, the CCR2+ IM subpopulation expanded, suggestive of circulating monocyte recruitment. Schistosoma exposure caused increased monocyte-recruitment ligand CCL2 expression in the resident FOLR2+ IM subpopulation. In contrast, the vascular pathology-driving protein TSP-1 was greatest in the CCR2+ IM subpopulation.
Conclusion: Schistosoma-induced PH involves crosstalk between IM subpopulations, with increased expression of monocyte recruitment ligands by resident FOLR2+ IMs, and the recruitment of CCR2+ IMs which express TSP-1 that activates TGF-β and causes PH.
Keywords: inflammation; macrophages; pulmonary hypertension; schistosomiasis; type 2 inflammation.
Copyright © 2024 Kumar, Kumar, Mickael, Fonseca Balladares, Nolan, Lee, Sanders, Nilsson, Molofsky, Tuder, Stenmark and Graham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

