Taenia solium cysticerci's extracellular vesicles Attenuate the AKT/mTORC1 pathway for Alleviating DSS-induced colitis in a murine model
- PMID: 38779712
- PMCID: PMC11112404
- DOI: 10.1002/jev2.12448
Taenia solium cysticerci's extracellular vesicles Attenuate the AKT/mTORC1 pathway for Alleviating DSS-induced colitis in a murine model
Abstract
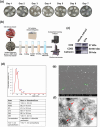
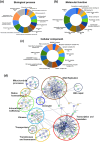
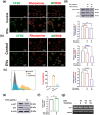
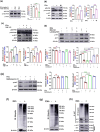
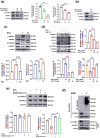
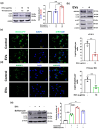
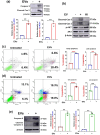
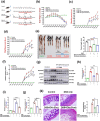
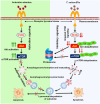
The excretory-secretory proteome plays a pivotal role in both intercellular communication during disease progression and immune escape mechanisms of various pathogens including cestode parasites like Taenia solium. The cysticerci of T. solium causes infection in the central nervous system known as neurocysticercosis (NCC), which affects a significant population in developing countries. Extracellular vesicles (EVs) are 30-150-nm-sized particles and constitute a significant part of the secretome. However, the role of EV in NCC pathogenesis remains undetermined. Here, for the first time, we report that EV from T. solium larvae is abundant in metabolites that can negatively regulate PI3K/AKT pathway, efficiently internalized by macrophages to induce AKT and mTOR degradation through auto-lysosomal route with a prominent increase in the ubiquitination of both proteins. This results in less ROS production and diminished bacterial killing capability among EV-treated macrophages. Due to this, both macro-autophagy and caspase-linked apoptosis are upregulated, with a reduction of the autophagy substrate sequestome 1. In summary, we report that T. solium EV from viable cysts attenuates the AKT-mTOR pathway thereby promoting apoptosis in macrophages, and this may exert immunosuppression during an early viable stage of the parasite in NCC, which is primarily asymptomatic. Further investigation on EV-mediated immune suppression revealed that the EV can protect the mice from DSS-induced colitis and improve colon architecture. These findings shed light on the previously unknown role of T. solium EV and the therapeutic role of their immune suppression potential.
Keywords: AKT; EVs; Taenia solium; apoptosis; autophagy; neurocysticercosis.
© 2024 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest concerning the work reported in this paper. None of the funders have any role in deciding/influencing the outcome of this study.
Figures

References
-
- Adalid‐Peralta, L. , Arce‐Sillas, A. , Fragoso, G. , Cardenas, G. , Rosetti, M. , Casanova‐Hernandez, D. , Rangel‐Escareño, C. , Uribe‐Figueroa, L. , Fleury, A. , & Sciutto, E. (2013). Cysticerci drive dendritic cells to promote in vitro and in vivo Tregs differentiation. Clinical & Developmental Immunology, 2013, 981468. 10.1155/2013/981468 - DOI - PMC - PubMed
-
- Amit, P. , Prasad, K. N. , Kumar, G. R. , Shweta, T. , Sanjeev, J. , Kumar, P. V. , & Mukesh, T. (2011). Immune response to different fractions of Taenia solium cyst fluid antigens in patients with neurocysticercosis. Experimental Parasitology, 127(3), 687–692. 10.1016/j.exppara.2010.11.006 - DOI - PubMed
-
- Ancarola, M. E. , Marcilla, A. , Herz, M. , Macchiaroli, N. , Perez, M. , Asurmendi, S. , Brehm, K. , Poncini, C. , Rosenzvit, M. , & Cucher, M. (2017). Cestode parasites release extracellular vesicles with microRNAs and immunodiagnostic protein cargo. International Journal for Parasitology, 47(10‐11), 675–686. 10.1016/j.ijpara.2017.05.003 - DOI - PubMed
-
- Arora, N. , Keshri, A. K. , Kaur, R. , Rawat, S. S. , Kumar, R. , Mishra, A. , & Prasad, A. (2023). Taenia solium excretory secretory proteins (ESPs) suppresses TLR4/AKT mediated ROS formation in human macrophages via hsa‐miR‐125. PLOS Neglected Tropical Diseases, 17(12), e0011858. 10.1371/journal.pntd.0011858 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

