Checkpoint inhibition enhances cell contacts between CD4+ T cells and Hodgkin-Reed-Sternberg cells of classic Hodgkin lymphoma
- PMID: 38779721
- PMCID: PMC11443406
- DOI: 10.3324/haematol.2023.284512
Checkpoint inhibition enhances cell contacts between CD4+ T cells and Hodgkin-Reed-Sternberg cells of classic Hodgkin lymphoma
Abstract
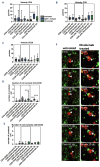
Although checkpoint molecules like CTLA-4 and PD1 have been described several years ago, checkpoint inhibitors such as nivolumab (an anti-PD-1 antibody) have only recently been used to treat classic Hodgkin lymphoma (cHL). Several studies have shown convincing therapeutic effects of nivolumab in cHL. However, the mechanism of action of nivolumab in cHL is not fully understood. The aim of this study was to monitor changes in cell motility and cell contacts after administration of nivolumab to an in vitro model of cHL as well as to native hyperplastic lymphoid tissue and native human tissue from cHL. In both tissue and in vitro, CD4+, CD8+, CD30+ and CD20+ cell velocities were unchanged after nivolumab incubation. In contrast, in primary cHL tissue, the duration of cell contacts between CD4+ T cells and Hodgkin-Reed-Sternberg cells was significantly increased after 5 hours of nivolumab treatment, and the number of contacts with HRS cells was also slightly increased for CD4+ T cells (not significant), suggesting that CD4+ T cells in particular contribute to the cytotoxicity observed as a result of nivolumab therapy. There was no change in the duration of cell contacts in the hyperplastic lymphoid tissue after nivolumab incubation. In conclusion, we show here for the first time by imaging of native lymphoma tissue an enhanced interaction of CD4+ T cells and Hodgkin-Reed-Sternberg cells in cHL after nivolumab administration.
Figures



References
-
- Balzano C, Buonavista N, Rouvier E, et al. . CTLA-4 and CD28: similar proteins, neighbouring genes. Int J Cancer Suppl. 1992;7:28-32. - PubMed
-
- Nishimura H, Nose M, Hiai H, et al. . Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141-151. - PubMed
-
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813-824. - PubMed
-
- Ott PA, Bang YJ, Piha-Paul SA, et al. . T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318-327. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

