ACVIM consensus statement on the treatment of immune thrombocytopenia in dogs and cats
- PMID: 38779941
- PMCID: PMC11256181
- DOI: 10.1111/jvim.17079
ACVIM consensus statement on the treatment of immune thrombocytopenia in dogs and cats
Abstract
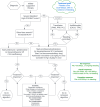
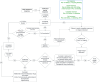
Management of immune thrombocytopenia (ITP) in dogs and cats is evolving, but there are no evidence-based guidelines to assist clinicians with treatment decisions. Likewise, the overall goals for treatment of ITP have not been established. Immunosuppressive doses of glucocorticoids are the first line treatment, but optimal treatment regimens beyond glucocorticoids remain uncertain. Additional options include secondary immunosuppressive drugs such as azathioprine, modified cyclosporine, and mycophenolate mofetil, usually selected based on clinician preference. Vincristine, human IV immunoglobulin (hIVIg), and transfusion of platelet or red blood cell-containing products are often used in more severe cases. Splenectomy and thrombopoietin receptor agonists are usually reserved for refractory cases, but when and in which patient these modalities should be employed is under debate. To develop evidence-based guidelines for individualized treatment of ITP patients, we asked 20 Population Intervention Comparison Outcome (PICO) format questions. These were addressed by 17 evidence evaluators using a literature pool of 288 articles identified by a structured search strategy. Evidence evaluators, using panel-designed templates and data extraction tools, summarized evidence and created guideline recommendations. These were integrated by treatment domain chairs and then refined by iterative Delphi survey review to reach consensus on the final guidelines. In addition, 19 non-PICO questions covering scenarios in which evidence was lacking or of low quality were answered by expert opinion using iterative Delphi surveys with panelist integration and refinement. Commentary was solicited from multiple relevant professional organizations before finalizing the consensus. The rigorous consensus process identified few comparative treatment studies, highlighting many areas of ITP treatment requiring additional studies. This statement is a companion manuscript to the ACVIM Consensus Statement on the Diagnosis of Immune Thrombocytopenia in Dogs and Cats.
Keywords: glucocorticoids; immunoglobulin; immunosuppressive; platelet; transfusion; vincristine.
© 2024 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Dana LeVine has received relevant research funding from American Kennel Club Canine Health Fund, BodeVet, Morris Animal Foundation, and the Veterinary & Comparative Clinical Immunology Society (VCCIS) and honoraria for speaking engagements from the ACVIM Forum, ECVIM‐CA Congress, ACVIM ACE, CVMA, and CanWest and is also a consultant for Cellphire Therapeutics. Robert Goggs has received relevant research funding from the American Kennel Club Canine Health Fund, Aurin Biotech, BodeVet, Morris Animal Foundation, and Okava Pharmaceuticals and honoraria for speaking engagements from ACVIM Forum, EVECC Congress, IVECCS, PacVet, UnisVet, and VCCIS. Barbara Kohn has held lectures, had research collaborations, and acted as a consultant for veterinary pharmaceutical and diagnostic companies. Linda Kidd was a Veterinary Field Specialist for Zoetis Diagnostics August 2022 to July 2023 and is an Associate Editor of the
Figures

References
-
- Harrington W, Minnich V, Hollingsworth J, Moore C. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38:1‐10. - PubMed
-
- Olsson B, Andersson PO, Jernås M, et al. T‐cell‐mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123‐1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
