Sulfoquinovosyl diacylglycerol is required for dimerisation of the Rhodobacter sphaeroides reaction centre-light harvesting 1 core complex
- PMID: 38780411
- PMCID: PMC11346425
- DOI: 10.1042/BCJ20240125
Sulfoquinovosyl diacylglycerol is required for dimerisation of the Rhodobacter sphaeroides reaction centre-light harvesting 1 core complex
Abstract
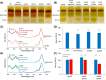
The reaction centre-light harvesting 1 (RC-LH1) core complex is indispensable for anoxygenic photosynthesis. In the purple bacterium Rhodobacter (Rba.) sphaeroides RC-LH1 is produced both as a monomer, in which 14 LH1 subunits form a C-shaped antenna around 1 RC, and as a dimer, where 28 LH1 subunits form an S-shaped antenna surrounding 2 RCs. Alongside the five RC and LH1 subunits, an additional polypeptide known as PufX provides an interface for dimerisation and also prevents LH1 ring closure, introducing a channel for quinone exchange that is essential for photoheterotrophic growth. Structures of Rba. sphaeroides RC-LH1 complexes revealed several new components; protein-Y, which helps to form the quinone channel; protein-Z, of unknown function and seemingly unique to dimers; and a tightly bound sulfoquinovosyl diacylglycerol (SQDG) lipid that interacts with two PufX arginine residues. This lipid lies at the dimer interface alongside weak density for a second molecule, previously proposed to be an ornithine lipid. In this work we have generated strains of Rba. sphaeroides lacking protein-Y, protein-Z, SQDG or ornithine lipids to assess the roles of these previously unknown components in the assembly and activity of RC-LH1. We show that whilst the removal of either protein-Y, protein-Z or ornithine lipids has only subtle effects, SQDG is essential for the formation of RC-LH1 dimers but its absence has no functional effect on the monomeric complex.
Keywords: Rhodobacter sphaeroides; RC-LH1; light harvesting; lipids; photosynthesis; reaction centre.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Cross-species investigation of the functions of the Rhodobacter PufX polypeptide and the composition of the RC-LH1 core complex.Biochim Biophys Acta. 2012 Feb;1817(2):336-52. doi: 10.1016/j.bbabio.2011.10.009. Epub 2011 Nov 3. Biochim Biophys Acta. 2012. PMID: 22079525
-
Dimerisation of the Rhodobacter sphaeroides RC-LH1 photosynthetic complex is not facilitated by a GxxxG motif in the PufX polypeptide.Biochim Biophys Acta. 2010 Nov;1797(11):1812-9. doi: 10.1016/j.bbabio.2010.07.007. Epub 2010 Jul 17. Biochim Biophys Acta. 2010. PMID: 20646993
-
Cryo-EM structure of the monomeric Rhodobacter sphaeroides RC-LH1 core complex at 2.5 Å.Biochem J. 2021 Oct 29;478(20):3775-3790. doi: 10.1042/BCJ20210631. Biochem J. 2021. PMID: 34590677 Free PMC article.
-
A comparative look at structural variation among RC-LH1 'Core' complexes present in anoxygenic phototrophic bacteria.Photosynth Res. 2020 Aug;145(2):83-96. doi: 10.1007/s11120-020-00758-3. Epub 2020 May 19. Photosynth Res. 2020. PMID: 32430765 Free PMC article. Review.
-
Structural and functional proteomics of intracytoplasmic membrane assembly in Rhodobacter sphaeroides.J Mol Microbiol Biotechnol. 2013;23(1-2):48-62. doi: 10.1159/000346520. Epub 2013 Apr 18. J Mol Microbiol Biotechnol. 2013. PMID: 23615195 Review.
Cited by
-
Anaerobic Faecalicatena spp. degrade sulfoquinovose via a bifurcated 6-deoxy-6-sulfofructose transketolase/transaldolase pathway to both C2- and C3-sulfonate intermediates.Front Microbiol. 2024 Dec 5;15:1491101. doi: 10.3389/fmicb.2024.1491101. eCollection 2024. Front Microbiol. 2024. PMID: 39712897 Free PMC article.
References
-
- Tucker, J.D., Siebert, C.A., Escalante, M., Adams, P.G., Olsen, J.D., Otto, C.et al. (2010) Membrane invagination in Rhodobacter sphaeroides is initiated at curved regions of the cytoplasmic membrane, then forms both budded and fully detached spherical vesicles. Mol. Microbiol. 76, 833–847 10.1111/j.1365-2958.2010.07153.x - DOI - PubMed
-
- Cartron, M.L., Olsen, J.D., Sener, M., Jackson, P.J., Brindley, A.A., Qian, P.et al. (2014) Integration of energy and electron transfer processes in the photosynthetic membrane of Rhodobacter sphaeroides. Biochim. Biophys. Acta - Bioenerg. 1837, 1769–1780 10.1016/j.bbabio.2014.02.003 - DOI - PMC - PubMed
-
- Swainsbury, D.J.K., Hawkings, F.R., Martin, E.C., Musiał, S., Salisbury, J.H., Jackson, P.J.et al. (2023) Cryo-EM structure of the four-subunit Rhodobacter sphaeroides cytochrome bc1 complex in styrene maleic acid nanodiscs. Proc. Natl Acad. Sci. U.S.A. 120, e2217922120 10.1073/pnas.2217922120 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

