Effectiveness and Safety of Ustekinumab in Pediatric Ulcerative Colitis: A Multi-center Retrospective Study from the Pediatric IBD Porto Group of ESPGHAN
- PMID: 38780740
- PMCID: PMC11335845
- DOI: 10.1007/s40272-024-00631-z
Effectiveness and Safety of Ustekinumab in Pediatric Ulcerative Colitis: A Multi-center Retrospective Study from the Pediatric IBD Porto Group of ESPGHAN
Abstract
Background and objectives: Current data on ustekinumab therapy in children with ulcerative colitis (UC) or unclassified inflammatory bowel disease (IBDU) are limited. We aimed to evaluate the effectiveness and safety of ustekinumab in pediatric UC and IBDU.
Methods: This multicenter retrospective study included 16 centers affiliated with the IBD Interest and Porto groups of ESPGHAN. Children with UC or IBDU treated with ustekinumab were enrolled. Demographic, clinical, laboratory, endoscopic, and imaging data as well as adverse events were recorded. Analyses were all based on the intention-to-treat principle.
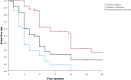
Results: Fifty-eight children (39 UC and 19 IBDU, median age 14.5 [IQR 11.5-16.5] years) were included. All had failed biologic therapies, and 38 (66%) had failed two or more biologics. Corticosteroid-free clinical remission (CFR) was observed in 27 (47%), 33 (57%), and 37 (64%) children at 16, 26, and 52 weeks, respectively. Normalization of C-reactive protein and calprotectin < 150 μg/g were achieved in 60% and 52%, respectively, by 52 weeks. Endoscopic and radiologic remissions were reached in 8% and 23%, respectively. The main predictors of CFR were diagnosis of UC compared with IBDU (hazard ratio [HR] 2.2, 95% CI 1.03-4.85; p = 0.041) and no prior vedolizumab therapy (HR 2.1, 95% CI 1.11-4.27; p = 0.023). Ustekinumab serum levels were not associated with disease activity. Adverse events were recorded in six (10%) children, leading to discontinuation of the drug in three.
Conclusion: Based on these findings, ustekinumab appears as an effective therapy for pediatric refractory UC and IBDU. The potential efficacy should be weighed against the risks of serious adverse events.
© 2024. The Author(s).
Conflict of interest statement
In the last 3 years DT received consultation fees, research grants, royalties, or honorarium from Janssen, Pfizer, Shaare Zedek Medical Center, Hospital for Sick Children, Ferring, Abbvie, Takeda, Prometheus Biosciences, Lilly, Roche, ThermoFisher, BMS, SorrisoPharma. KLK has received consultation fees from Abbvie, Biocodexa and Tillotts Pharma and research grants from Pediatric Research Foundation (Finland) and Helsinki University Hospital Research Fund. In the past 3 years, Ben Kang has recieved speaker fees, consultation fees, or research grants from Celltrion, Janssen, Abbvie, Takeda, Yuhan, Yungjin, JW Pharmaceutical, and Samsung Bioepis.
Figures
References
-
- Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257–91. 10.1097/MPG.0000000000002035 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials