ISG15/GRAIL1/CD3 axis influences survival of patients with esophageal adenocarcinoma
- PMID: 38781019
- PMCID: PMC11383178
- DOI: 10.1172/jci.insight.179315
ISG15/GRAIL1/CD3 axis influences survival of patients with esophageal adenocarcinoma
Abstract
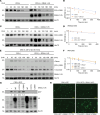
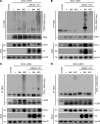
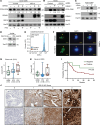
Immunosuppression is a common feature of esophageal adenocarcinoma (EAC) and has been linked to poor overall survival (OS). We hypothesized that upstream factors might negatively influence CD3 levels and T cell activity, thus promoting immunosuppression and worse survival. We used clinical data and patient samples of those who progressed from Barrett's to dysplasia to EAC, investigated gene (RNA-Seq) and protein (tissue microarray) expression, and performed cell biology studies to delineate a pathway impacting CD3 protein stability that might influence EAC outcome. We showed that the loss of both CD3-ε expression and CD3+ T cell number correlated with worse OS in EAC. The gene related to anergy in lymphocytes isoform 1 (GRAIL1), which is the prominent isoform in EACs, degraded (ε, γ, δ) CD3s and inactivated T cells. In contrast, isoform 2 (GRAIL2), which is reduced in EACs, stabilized CD3s. Further, GRAIL1-mediated CD3 degradation was facilitated by interferon-stimulated gene 15 (ISG15), a ubiquitin-like protein. Consequently, the overexpression of a ligase-dead GRAIL1, ISG15 knockdown, or the overexpression of a conjugation-defective ISG15-leucine-arginine-glycine-glycine mutant could increase CD3 levels. Together, we identified an ISG15/GRAIL1/mutant p53 amplification loop negatively influencing CD3 levels and T cell activity, thus promoting immunosuppression in EAC.
Keywords: Cancer; Gastroenterology; Immunology.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

