Astrocyte-to-microglia communication via Sema4B-Plexin-B2 modulates injury-induced reactivity of microglia
- PMID: 38781210
- PMCID: PMC11145257
- DOI: 10.1073/pnas.2400648121
Astrocyte-to-microglia communication via Sema4B-Plexin-B2 modulates injury-induced reactivity of microglia
Abstract
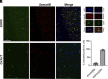
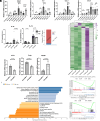
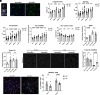
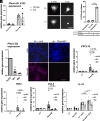
After central nervous system injury, a rapid cellular and molecular response is induced. This response can be both beneficial and detrimental to neuronal survival in the first few days and increases the risk for neurodegeneration if persistent. Semaphorin4B (Sema4B), a transmembrane protein primarily expressed by cortical astrocytes, has been shown to play a role in neuronal cell death following injury. Our study shows that after cortical stab wound injury, cytokine expression is attenuated in Sema4B-/- mice, and microglia/macrophage reactivity is altered. In vitro, Sema4B enhances the reactivity of microglia following injury, suggesting astrocytic Sema4B functions as a ligand. Moreover, injury-induced microglia reactivity is attenuated in the presence of Sema4B-/- astrocytes compared to Sema4B+/- astrocytes. In vitro experiments indicate that Plexin-B2 is the Sema4B receptor on microglia. Consistent with this, in microglia/macrophage-specific Plexin-B2-/- mice, similar to Sema4B-/- mice, microglial/macrophage reactivity and neuronal cell death are attenuated after cortical injury. Finally, in Sema4B/Plexin-B2 double heterozygous mice, microglial/macrophage reactivity is also reduced after injury, supporting the idea that both Sema4B and Plexin-B2 are part of the same signaling pathway. Taken together, we propose a model in which following injury, astrocytic Sema4B enhances the response of microglia/macrophages via Plexin-B2, leading to increased reactivity.
Keywords: Plexin-B2; Sema4B; astrocyte; inflammation; microglia.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

