Foraging mechanisms in excavate flagellates shed light on the functional ecology of early eukaryotes
- PMID: 38781211
- PMCID: PMC11145212
- DOI: 10.1073/pnas.2317264121
Foraging mechanisms in excavate flagellates shed light on the functional ecology of early eukaryotes
Abstract
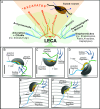
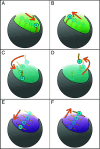
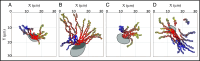
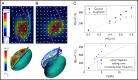
The phagotrophic flagellates described as "typical excavates" have been hypothesized to be morphologically similar to the Last Eukaryotic Common Ancestor and understanding the functional ecology of excavates may therefore help shed light on the ecology of these early eukaryotes. Typical excavates are characterized by a posterior flagellum equipped with a vane that beats in a ventral groove. Here, we combined flow visualization and observations of prey capture in representatives of the three clades of excavates with computational fluid dynamic modeling, to understand the functional significance of this cell architecture. We record substantial differences amongst species in the orientation of the vane and the beat plane of the posterior flagellum. Clearance rate magnitudes estimated from flow visualization and modeling are both like that of other similarly sized flagellates. The interaction between a vaned flagellum beating in a confinement is modeled to produce a very efficient feeding current at low energy costs, irrespective of the beat plane and vane orientation and of all other morphological variations. Given this predicted uniformity of function, we suggest that the foraging systems of typical excavates studied here may be good proxies to understand those potentially used by our distant ancestors more than 1 billion years ago.
Keywords: clearance rate; early eukaryotic evolution; feeding current; prey capture; vane-bearing flagella.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
Novel Foraging Mechanisms in Atypical Excavate Flagellates.J Eukaryot Microbiol. 2025 May-Jun;72(3):e70010. doi: 10.1111/jeu.70010. J Eukaryot Microbiol. 2025. PMID: 40247630 Free PMC article.
-
The function of the feeding groove of 'typical excavate' flagellates.J Eukaryot Microbiol. 2024 Mar-Apr;71(2):e13016. doi: 10.1111/jeu.13016. Epub 2023 Dec 18. J Eukaryot Microbiol. 2024. PMID: 38108228
-
Flagellar beat patterns and their possible evolution.Biosystems. 1981;14(3-4):423-31. doi: 10.1016/0303-2647(81)90047-2. Biosystems. 1981. PMID: 7337816
-
Alternative cytoskeletal landscapes: cytoskeletal novelty and evolution in basal excavate protists.Curr Opin Cell Biol. 2013 Feb;25(1):134-41. doi: 10.1016/j.ceb.2012.11.005. Epub 2013 Jan 8. Curr Opin Cell Biol. 2013. PMID: 23312067 Free PMC article. Review.
-
Predation in a Microbial World: Mechanisms and Trade-Offs of Flagellate Foraging.Ann Rev Mar Sci. 2024 Jan 17;16:361-381. doi: 10.1146/annurev-marine-020123-102001. Epub 2023 Jun 27. Ann Rev Mar Sci. 2024. PMID: 37368955 Review.
Cited by
-
A Deeply Branching Lineage in Heterolobosea (Discoba) With Multiflagellated and Multigrooved Life Stages.J Eukaryot Microbiol. 2025 Sep-Oct;72(5):e70037. doi: 10.1111/jeu.70037. J Eukaryot Microbiol. 2025. PMID: 40770464 Free PMC article.
-
A diverse Palaeoproterozoic microbial ecosystem implies early eukaryogenesis.Philos Trans R Soc Lond B Biol Sci. 2025 Aug 7;380(1931):20240092. doi: 10.1098/rstb.2024.0092. Epub 2025 Aug 7. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40770989 Free PMC article.
-
Novel Foraging Mechanisms in Atypical Excavate Flagellates.J Eukaryot Microbiol. 2025 May-Jun;72(3):e70010. doi: 10.1111/jeu.70010. J Eukaryot Microbiol. 2025. PMID: 40247630 Free PMC article.
References
-
- Fenchel T., Ecology of heterotrophic microflagellates. IV quantitative occurrence and importance as bacterial consumers. Mar. Ecol. Prog. Ser. 9, 35–42 (1982).
-
- Tikhonenkov D. V., Predatory flagellates–The new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance. Protistology 14, 15–22 (2020).
-
- Kiørboe T., Fluid dynamic constraints on resource acquisition in small pelagic organisms. Eur. Phys. J. Spec. Top 225, 669–683 (2016).
-
- Edwards K. F., Li Q., Steward G. F., Ingestion kinetics of mixotrophic and heterotrophic flagellates. Limnol. Oceanogr. 68, 917–927 (2023).
-
- Eglit Y., et al. , Meteora sporadica, a protist with incredible cell architecture, is related to Hemimastigophora. Curr. Biol. 34, 451–459.e6 (2024). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

