Acalabrutinib plus venetoclax and rituximab in treatment-naive mantle cell lymphoma: 2-year safety and efficacy analysis
- PMID: 38781315
- PMCID: PMC11399641
- DOI: 10.1182/bloodadvances.2023012424
Acalabrutinib plus venetoclax and rituximab in treatment-naive mantle cell lymphoma: 2-year safety and efficacy analysis
Abstract
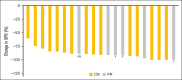
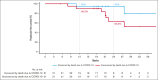
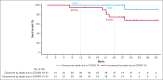
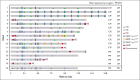
This phase 1b study evaluated safety and efficacy of acalabrutinib, venetoclax, and rituximab (AVR) in treatment-naive mantle cell lymphoma (TN MCL). Patients received acalabrutinib from cycle 1 until progressive disease (PD) or undue toxicity, rituximab for 6 cycles with maintenance every other cycle through cycle 24 or until PD, and venetoclax, beginning at cycle 2, for 24 cycles. Twenty-one patients were enrolled; 95.2% completed induction (6 AVR cycles) and 47.6% continued acalabrutinib maintenance. Thirteen (61.9%) patients had grade 3-4 adverse events (AEs), most commonly neutropenia (33.3%). Seven (33.3%) patients had COVID-19 infection (6 [28.6%] serious AEs and 5 [23.8%] deaths, all among unvaccinated patients). There was no grade ≥3 atrial fibrillation, ventricular tachyarrhythmias, major hemorrhages, or tumor lysis syndrome. Overall response rate (ORR) was 100% (95% CI, 83.9-100.0) with 71.4% complete response. With median follow-up of 27.8 months, median progression-free survival (PFS) and overall survival (OS) were not reached. PFS rates at 1 and 2 years were 90.5% (95% CI, 67.0-97.5) and 63.2% (95% CI, 34.7-82.0), respectively; both were 95% after censoring COVID-19 deaths. OS rates at 1 and 2 years were 95.2% (95% CI, 70.7-99.3) and 75.2% (95% CI, 50.3-88.9), respectively; both were 100% after censoring COVID-19 deaths. Overall, 87.5% of patients with available minimal residual disease (MRD) data achieved MRD negativity (10-6; next-generation sequencing) during treatment. AVR represents a chemotherapy-free regimen for TN MCL and resulted in high ORR and high rates of MRD negativity. The trial was registered at www.ClinicalTrials.gov as #NCT02717624.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.W. reports consultancy from AbbVie, Acerta Pharma, ADC Therapeutics America, Amphista Therapeutics Limited, AstraZeneca, Be Biopharma, BeiGene, BioInvent, Bristol Myers Squibb (BMS), Deciphera, DTRM Biopharma (Cayman) Limited, Genentech, InnoCare, Janssen, Kite Pharma, Leukemia & Lymphoma Society, Lilly, Merck, Miltenyi Biomedicine, Milken Institute, Oncternal, Parexel, Pepromene Bio, Pharmacyclics, and VelosBio; research funding from Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genmab, Genentech, InnoCare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, VelosBio, and Vincerx; and honoraria from AbbVie, Acerta Pharma, AstraZeneca, Bantam Pharmaceutical, BeiGene, BioInvent, BMS, CAHON, Catamount Medical Education, Dava Oncology, Eastern Virginia Medical School, Genmab, i3 Health, IDEOlogy Health, Janssen, Kite Pharma, Leukemia & Lymphoma Society, Medscape, Meeting Minds Experts, MD Education, MJH Life Sciences, Merck, Moffit Cancer Center, MSC National Research Institute of Oncology, National Institutes of Health, Nurix, Oncology Specialty Group, OncLive, Pharmacyclics, Physicians Education Resources, Practice Point Communications, Research To Practice, Scripps, Syneos Health, Studio ER Congressi, South African Clinical Hematology Society, and WebMD. T.R. reports research funding from Acerta, Roche, Janssen, AbbVie, Novartis, BioGene, AstraZeneca, Pharmacyclics, Pfizer, MorphoSys, UTX-TGR, GlaxoSmithKline, and BMS; travel, accommodation, and expenses from Roche, Janssen, and AbbVie; honoraria from Sandoz, Novartis, Octapharma, BioGene, AstraZeneca, and Pharmacyclics; and consultancy from Sandoz, Takeda, and Momenta. K.J.M. reports research funding from Pharmacyclics, Pfizer, and BMS; and consulting/advisory for AbbVie, ADC, Acerta, AstraZeneca, BeiGene, BMS, Celgene, Genmab, Genentech, Gilead, Incyte, Janssen, Kite, Lilly, MorphoSys, and Pharmacyclics. T.P. reports consultancy from AbbVie, ADCT, AstraZeneca, Bayer, BeiGene, BMS, Genmab, Genentech, Epizyme, Eli Lilly, Incyte, Janssen, Kite Pharma, MorphoSys, Pharmacyclics, Seattle Genetics, Regeneron, and Xencor; research funding from AbbVie, Bayer, Genentech, and Sobi; served as scientific committee member for Genmab, Genentech, and Merck. S.D.S. reports research funding from ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, BMS (spouse), De Novo Biopharma, Enterome, Genentech, Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck Sharp and Dohme Corp, MorphoSys, Nanjing Pharmaceuticals Co, Ltd, and Viracta Therapeutics; consultancy/advisory board roles for ADC Therapeutics, BeiGene, Kite Pharma, Incyte, Numab Therapeutics AG, AbbVie, and Coherus BioSciences; and advisory board (spouse) for Genentech. R.C. is employed by and holds stock ownership of AstraZeneca. C.-C.W. is employed by AstraZeneca. V.M. is employed by and holds stock ownership of AstraZeneca. A family member is an employee and stockholder of Gilead Sciences. W.J. reports research funding and advisory boards for AstraZeneca. D.G. declares no competing financial interests.
Figures


References
-
- Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the second Nordic mantle cell lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175(3):410–418. - PubMed
-
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. - PubMed
-
- Csanádi M, Ágh T, Tordai A, Tapprich C, Vokó Z, Stamatopoulos K. Secondary primary malignancies after treatment with chemo-immunotherapy in treatment-naïve patients with CLL: a systematic literature review. Expert Rev Hematol. 2022;15(3):273–284. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

