Prevalence and Spectrum of AR Ligand-Binding Domain Mutations Detected in Circulating-Tumor DNA Across Disease States in Men With Metastatic Castration-Resistant Prostate Cancer
- PMID: 38781544
- PMCID: PMC11486455
- DOI: 10.1200/PO.23.00330
Prevalence and Spectrum of AR Ligand-Binding Domain Mutations Detected in Circulating-Tumor DNA Across Disease States in Men With Metastatic Castration-Resistant Prostate Cancer
Abstract
Purpose: Metastatic castration-resistant prostate cancer (mCRPC) is typically treated with agents directly or indirectly targeting the androgen receptor (AR) pathway. However, such treatment is limited by resistance mechanisms, including the development of activating mutations in the AR ligand-binding domain (AR-LBD).
Methods: This study evaluated a database of over 15,000 patients with advanced prostate cancer (PC) undergoing comprehensive circulating-tumor DNA analysis (Guardant360, Redwood City, CA) between 2014 and 2021, with associated clinical information from administrative claims (GuardantINFORM database).
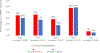
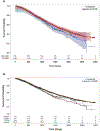
Results: Of 15,705 patients with PC included, 54% had mCRPC at the time of their blood draw. Of those, 49% had previous treatment with an AR pathway inhibitor (ARPi). AR-LBD mutation prevalence was 15% in patients with mCRPC who were untreated with a next-generation ARPi, 22% in those after one line of ARPi therapy, and 24% in those after two lines of ARPi treatment. Next-generation ARPi treatment yielded an increase in AR L702H and T878A/S mutations after abiraterone, and an increase in AR L702H and F877L mutations after enzalutamide. AR-LBD+ patients demonstrated unique biology, including increased concurrent mutations in the cell-cycle, wingless-related integration site, homologous recombination repair, and phospho-inositide 3-kinase pathways (all P < .0005), and greater low-level (copy number <10) AR amplifications (P = .0041). AR-LBD+ patients exhibited worse overall survival (OS) relative to a matched cohort of AR-LBD- patients (50.1 v 60.7 months, unadjusted log-rank P = .013).
Conclusion: This large database analysis demonstrates that AR-LBD mutation prevalence increases after next-generation ARPi use. AR-LBD+ tumors demonstrate unique biology (more oncogenic pathway mutations and low-level AR amplification) and reduced OS. These findings inform the development of novel therapies designed to circumvent AR-mediated therapeutic resistance.
Conflict of interest statement
Conflicts of Interest
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

