Phase 1 study of CAR-37 T cells in patients with relapsed or refractory CD37+ lymphoid malignancies
- PMID: 38781564
- PMCID: PMC11830985
- DOI: 10.1182/blood.2024024104
Phase 1 study of CAR-37 T cells in patients with relapsed or refractory CD37+ lymphoid malignancies
Abstract
We report a first-in-human clinical trial using chimeric antigen receptor (CAR) T cells targeting CD37, an antigen highly expressed in B- and T-cell malignancies. Five patients with relapsed or refractory CD37+ lymphoid malignancies were enrolled and infused with autologous CAR-37 T cells. CAR-37 T cells expanded in the peripheral blood of all patients and, at peak, comprised >94% of the total lymphocytes in 4 of 5 patients. Tumor responses were observed in 4 of 5 patients with 3 complete responses, 1 mixed response, and 1 patient whose disease progressed rapidly and with relative loss of CD37 expression. Three patients experienced prolonged and severe pancytopenia, and in 2 of these patients, efforts to ablate CAR-37 T cells, which were engineered to coexpress truncated epidermal growth factor receptor, with cetuximab were unsuccessful. Hematopoiesis was restored in these 2 patients after allogeneic hematopoietic stem cell transplantation. No other severe, nonhematopoietic toxicities occurred. We investigated the mechanisms of profound pancytopenia and did not observe activation of CAR-37 T cells in response to hematopoietic stem cells in vitro or hematotoxicity in humanized models. Patients with pancytopenia had sustained high levels of interleukin-18 (IL-18) with low levels of IL-18 binding protein in their peripheral blood. IL-18 levels were significantly higher in CAR-37-treated patients than in both cytopenic and noncytopenic cohorts of CAR-19-treated patients. In conclusion, CAR-37 T cells exhibited antitumor activity, with significant CAR expansion and cytokine production. CAR-37 T cells may be an effective therapy in hematologic malignancies as a bridge to hematopoietic stem cell transplant. This trial was registered at www.ClinicalTrials.gov as #NCT04136275.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflicts-of-interest disclosure: M.V.M. and I.S. report being inventors on patents describing chimeric antigen receptor-37 (CAR-37) therapy titled “CD37-Targeted Chimeric Antigen Receptor T Cells for Lymphomas/Leukemias” (PCT/US2018/022974); “Anti-CD37 Chimeric Antigen Receptor T Cells for Non-Hodgkin Lymphoma” (PCT/US2019/038518). In addition, M.V.M. reports being an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital and the University of Pennsylvania (some licensed to Novartis). M.V.M. reports holding equity in AffyImmune, Century Therapeutics, Oncternal Therapeutics, Neximmune, and TCR2; serving on the board of directors of 2seventy bio; and has served as a consultant for multiple companies involved in cell therapies. M.V.M.’s interests were reviewed and are managed by the Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. J.F.D. reports holding equity in Magenta Therapeutics and Wugen Inc; receiving research support from MacroGenics, Bioline, and Incyte; and serving as a consultant for Vertex, bluebird bio, SPARC, and RiverVest. J.R. reports receiving research funding from Equillium, Kite/Gilead, Novartis, and Oncternal Therapeutics; and serving on the scientific advisory boards of Akron Biotech, Clade Therapeutics, Garuda, LifeVault Bio, Novartis, Smart Immune, and TScan Therapeutics. The remaining authors declare no competing financial interests.
Figures


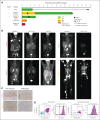

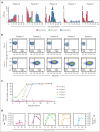

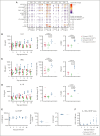
Comment in
-
Anti-CD37 CAR T cells: another arrow in the quiver.Blood. 2024 Sep 12;144(11):1133-1134. doi: 10.1182/blood.2024025361. Blood. 2024. PMID: 39264608 No abstract available.
References
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

