Iron accumulation in ovarian microenvironment damages the local redox balance and oocyte quality in aging mice
- PMID: 38781731
- PMCID: PMC11145558
- DOI: 10.1016/j.redox.2024.103195
Iron accumulation in ovarian microenvironment damages the local redox balance and oocyte quality in aging mice
Abstract
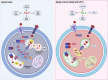
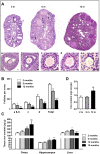
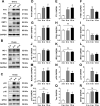
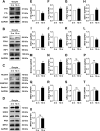
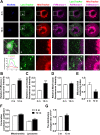
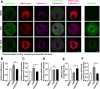
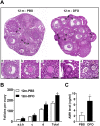
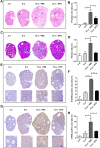
Accumulating oxidative damage is a primary driver of ovarian reserve decline along with aging. However, the mechanism behind the imbalance in reactive oxygen species (ROS) is not yet fully understood. Here we investigated changes in iron metabolism and its relationship with ROS disorder in aging ovaries of mice. We found increased iron content in aging ovaries and oocytes, along with abnormal expression of iron metabolic proteins, including heme oxygenase 1 (HO-1), ferritin heavy chain (FTH), ferritin light chain (FTL), mitochondrial ferritin (FTMT), divalent metal transporter 1 (DMT1), ferroportin1(FPN1), iron regulatory proteins (IRP1 and IRP2) and transferrin receptor 1 (TFR1). Notably, aging oocytes exhibited enhanced ferritinophagy and mitophagy, and consistently, there was an increase in cytosolic Fe2+, elevated lipid peroxidation, mitochondrial dysfunction, and augmented lysosome activity. Additionally, the ovarian expression of p53, p21, p16 and microtubule-associated protein tau (Tau) were also found to be upregulated. These alterations could be phenocopied with in vitro Fe2+ administration in oocytes from 2-month-old mice but were alleviated by deferoxamine (DFO). In vivo application of DFO improved ovarian iron metabolism and redox status in 12-month-old mice, and corrected the alterations in cytosolic Fe2+, ferritinophagy and mitophagy, as well as related degenerative changes in oocytes. Thereby in the whole, DFO delayed the decline in ovarian reserve and significantly increased the number of superovulated oocytes with reduced fragmentation and aneuploidy. Together, our findings suggest that aging-related disturbance in ovarian iron homeostasis contributes to excessive ROS production and that iron chelation may improve ovarian redox status, and efficiently delay the decline in ovarian reserve and oocyte quality in aging mice. These data propose a novel intervention strategy for preserving the ovarian reserve function in elderly women.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Wang S., Zheng Y., Li J., Yu Y., Zhang W., Song M., Liu Z., Min Z., Hu H., Jing Y., He X., Sun L., Ma L., Esteban C.R., Chan P., Qiao J., Zhou Q., Izpisua Belmonte J.C., Qu J., Tang F., Liu G.H. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180(3):585–600. - PubMed
-
- Li Q., Geng X., Zheng W., Tang J., Xu B., Shi Q. Current understanding of ovarian aging. Sci. China Life Sci. 2012;55(8):659–669. - PubMed
-
- Ahmed T.A., Ahmed S.M., El-Gammal Z., Shouman S., Ahmed A., Mansour R., El-Badri N. Oocyte aging: the role of cellular and environmental factors and impact on female fertility. Adv. Exp. Med. Biol. 2020;1247:109–123. - PubMed
-
- Wang L., Tang J., Wang L., Tan F., Song H., Zhou J., Li F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021;236(12):7966–7983. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

