SARS-CoV-2 Disease Severity and Cycle Threshold Values in Children Infected during Pre-Delta, Delta, and Omicron Periods, Colorado, USA, 2021-2022
- PMID: 38781929
- PMCID: PMC11139003
- DOI: 10.3201/eid3006.231427
SARS-CoV-2 Disease Severity and Cycle Threshold Values in Children Infected during Pre-Delta, Delta, and Omicron Periods, Colorado, USA, 2021-2022
Abstract
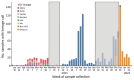
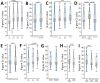
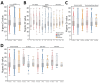
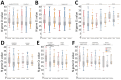
In adults, viral load and disease severity can differ by SARS-CoV-2 variant, patterns less understood in children. We evaluated symptomatology, cycle threshold (Ct) values, and SARS-CoV-2 variants among 2,299 pediatric SARS-CoV-2 patients (0-21 years of age) in Colorado, USA, to determine whether children infected with Delta or Omicron had different symptom severity or Ct values than during earlier variants. Children infected during the Delta and Omicron periods had lower Ct values than those infected during pre-Delta, and children <1 year of age had lower Ct values than older children. Hospitalized symptomatic children had lower Ct values than asymptomatic patients. Compared with pre-Delta, more children infected during Delta and Omicron were symptomatic (75.4% pre-Delta, 95.3% Delta, 99.5% Omicron), admitted to intensive care (18.8% pre-Delta, 39.5% Delta, 22.9% Omicron), or received oxygen support (42.0% pre-Delta, 66.3% Delta, 62.3% Omicron). Our data reinforce the need to include children, especially younger children, in pathogen surveillance efforts.
Keywords: COVID-19; COVID-19 pandemic; COVID-19 testing; Colorado; Ct values; Delta; Omicron; SARS-CoV-2; United States; children; coronavirus disease; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; whole-genome sequencing; zoonoses.
Figures
References
-
- Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, et al. ; COVID-19 Genomics UK (COG-UK) consortium. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–42. 10.1016/S1473-3099(21)00475-8 - DOI - PMC - PubMed
-
- Lambrou AS, Shirk P, Steele MK, Paul P, Paden CR, Cadwell B, et al. ; Strain Surveillance and Emerging Variants Bioinformatic Working Group; Strain Surveillance and Emerging Variants NS3 Working Group. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants - United States, June 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:206–11. 10.15585/mmwr.mm7106a4 - DOI - PMC - PubMed
-
- Taylor CA, Patel K, Pham H, Whitaker M, Anglin O, Kambhampati AK, et al. ; COVID-NET Surveillance Team. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance— COVID-NET, 14 states, January–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1513–9. 10.15585/mmwr.mm7043e1 - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

