Patterning and folding of intestinal villi by active mesenchymal dewetting
- PMID: 38781967
- PMCID: PMC11166531
- DOI: 10.1016/j.cell.2024.04.039
Patterning and folding of intestinal villi by active mesenchymal dewetting
Abstract
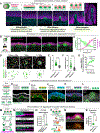
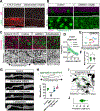
Tissue folds are structural motifs critical to organ function. In the intestine, bending of a flat epithelium into a periodic pattern of folds gives rise to villi, finger-like protrusions that enable nutrient absorption. However, the molecular and mechanical processes driving villus morphogenesis remain unclear. Here, we identify an active mechanical mechanism that simultaneously patterns and folds the intestinal epithelium to initiate villus formation. At the cellular level, we find that PDGFRA+ subepithelial mesenchymal cells generate myosin II-dependent forces sufficient to produce patterned curvature in neighboring tissue interfaces. This symmetry-breaking process requires altered cell and extracellular matrix interactions that are enabled by matrix metalloproteinase-mediated tissue fluidization. Computational models, together with in vitro and in vivo experiments, revealed that these cellular features manifest at the tissue level as differences in interfacial tensions that promote mesenchymal aggregation and interface bending through a process analogous to the active dewetting of a thin liquid film.
Keywords: Cahn-Hilliard; active fluids; biophysics; cell adhesion; development; extracellular matrix; morphogenesis; patterning; phase separation; self-organization.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Z.J.G. and C.S.M. hold patents related to the MULTI-seq barcoding method.
Figures




Update of
-
Patterning and folding of intestinal villi by active mesenchymal dewetting.bioRxiv [Preprint]. 2023 Aug 15:2023.06.25.546328. doi: 10.1101/2023.06.25.546328. bioRxiv. 2023. Update in: Cell. 2024 Jun 6;187(12):3072-3089.e20. doi: 10.1016/j.cell.2024.04.039. PMID: 37425793 Free PMC article. Updated. Preprint.
References
-
- Tallinen T, Chung JY, Rousseau F, Girard N, Lefèvre J, and Mahadevan L (2016). On the growth and form of cortical convolutions. Nat. Phys. 12, 588–593. 10.1038/nphys3632. - DOI
-
- Garikipati K (2017). Journal of the Mechanics and Physics of Solids Perspectives on the mathematics of biological patterning and morphogenesis. J. Mech. Phys. Solids 99, 192–210. 10.1016/j.jmps.2016.11.013. - DOI
-
- Harris AK, Stopak D, and Warner P (1984). Generation of spatially periodic patterns by a mechanical instability : a mechanical alternative to the Turing model. J Embryol Exp Morphol 20, 1–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

