The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia
- PMID: 38782077
- PMCID: PMC11410545
- DOI: 10.1016/j.bcp.2024.116298
The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia
Abstract
The intricate balance between excitation and inhibition (E/I) in the brain plays a crucial role in normative information processing. Dysfunctions in the E/I balance have been implicated in various psychiatric disorders, including schizophrenia (SCZ). In particular, abnormalities in GABAergic signaling, specifically in parvalbumin (PV)-containing interneurons, have been consistently observed in SCZ pathophysiology. PV interneuron function is vital for maintaining an ideal E/I balance, and alterations in PV interneuron-mediated inhibition contribute to circuit deficits observed in SCZ, including hippocampus hyperactivity and midbrain dopamine system overdrive. While current antipsychotic medications primarily target D2 dopamine receptors and are effective primarily in treating positive symptoms, novel therapeutic strategies aiming to restore the E/I balance could potentially mitigate not only positive symptoms but also negative symptoms and cognitive deficits. This could involve, for instance, increasing the inhibitory drive onto excitatory neurons or decreasing the putative enhanced pyramidal neuron activity due to functional loss of PV interneurons. Compounds targeting the glycine site at glutamate NMDA receptors and muscarinic acetylcholine receptors on PV interneurons that can increase PV interneuron drive, as well as drugs that increase the postsynaptic action of GABA, such as positive allosteric modulators of α5-GABA-A receptors, and decrease glutamatergic output, such as mGluR2/3 agonists, represent promising approaches. Preventive strategies aiming at E/I balance also represent a path to reduce the risk of transitioning to SCZ in high-risk individuals. Therefore, compounds with novel mechanisms targeting E/I balance provide optimism for more effective and tailored interventions in the management of SCZ.
Keywords: Excitatory-inhibitory balance; PV interneuron; Schizophrenia.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Daniela L. Uliana, Joao Roberto F. Lisboa and Felipe V. Gomes: no interest to disclosure. Anthony A. Grace has received consulting fees from Alkermes, Lundbeck, Takeda, Roche, and Lyra and Concert and research funding from Lundbeck, Newron, and Merck.
Figures


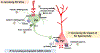
References
-
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K, Neocortical excitation/inhibition balance in information processing and social dysfunction, Nature 477 (2011) 171–178. 10.1038/nature10360. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

