True-Positive 18F-Flotufolastat Lesions in Patients with Prostate Cancer Recurrence with Baseline-Negative Conventional Imaging: Results from the Prospective, Phase 3, Multicenter SPOTLIGHT Study
- PMID: 38782456
- PMCID: PMC11218719
- DOI: 10.2967/jnumed.123.267271
True-Positive 18F-Flotufolastat Lesions in Patients with Prostate Cancer Recurrence with Baseline-Negative Conventional Imaging: Results from the Prospective, Phase 3, Multicenter SPOTLIGHT Study
Abstract
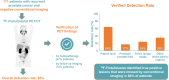
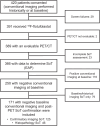
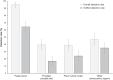
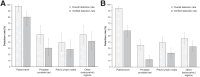
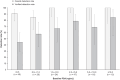
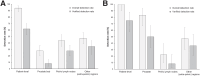
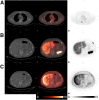
18F-rhPSMA-7.3 (18F-flotufolastat) is a high-affinity prostate-specific membrane antigen-targeted diagnostic radiopharmaceutical for PET imaging in patients with prostate cancer. Here, we report findings from the SPOTLIGHT study (NCT04186845), assessing the performance of 18F-flotufolastat PET/CT for identifying prostate-specific membrane antigen-positive lesions confirmed by standard of truth (SoT) in men with biochemical recurrence of prostate cancer and negative conventional imaging at baseline. Methods: Men with biochemical recurrence received 296 MBq of 18F-flotufolastat intravenously and then underwent PET/CT 50-70 min later. 18F-flotufolastat PET/CT findings were evaluated by 3 masked central readers and verified using histopathology or follow-up confirmatory imaging (CT, MRI, bone scan, or 18F-fluciclovine PET/CT) as the SoT. The present analysis evaluated all patients who had negative conventional imaging at baseline, underwent 18F-flotufolastat PET/CT, and had SoT verification by histopathology or follow-up confirmatory imaging to report detection rate (DR), which is the number of patients with at least 1 PET-positive lesion, divided by the number of evaluable patients, and verified DR (VDR), which is the proportion of patients with at least 1 true-positive lesion as verified by SoT, of all patients scanned (PET-positive and PET-negative scans). DR and VDR were calculated and stratified according to prior therapy. Majority read data (agreement between ≥2 readers) are reported. Results: In total, 171 patients with negative baseline conventional imaging and SoT by histopathology or post-PET confirmatory imaging were evaluated. By majority read, the overall 18F-flotufolastat DR among these patients was 95% (163/171; 95% CI, 91.0%-98.0%), and 110 of 171 of these patients had at least 1 true-positive lesion identified (VDR, 64%; 95% CI, 56.7%-71.5%). In the postprostatectomy group (133/171), 8.3% of patients had at least 1 true-positive lesion in the prostate bed, 28% in pelvic lymph nodes, and 35% in other sites. Among those who had received radiotherapy (36/171), 50% of patients had true-positive detections in the prostate, 8.3% in pelvic lymph nodes, and 36% in other sites. Conclusion: 18F-flotufolastat frequently identified true-positive prostate cancer lesions in patients with negative conventional imaging. 18F-flotufolastat may help to better define sites of disease recurrence and inform salvage therapy decisions than does conventional imaging, potentially leading to improved outcomes.
Keywords: 18F-flotufolastat; PSMA; biochemical recurrence; prostate cancer; rhPSMA.
© 2024 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
18F-Flotufolastat Positron Emission Tomography in African American Patients With Suspected Prostate Cancer Recurrence: Findings From the Phase 3 SPOTLIGHT Study.Adv Radiat Oncol. 2024 Jul 14;9(9):101571. doi: 10.1016/j.adro.2024.101571. eCollection 2024 Sep. Adv Radiat Oncol. 2024. PMID: 39188996 Free PMC article.
-
Biochemical failure-free survival of 18F-rhPSMA-7 and 18F-flotufolastat PET-guided salvage radiotherapy for patients with recurrent prostate cancer.Sci Rep. 2025 Jan 17;15(1):2234. doi: 10.1038/s41598-024-83074-3. Sci Rep. 2025. PMID: 39824988 Free PMC article.
-
68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Sep;4(5):686-693. doi: 10.1016/j.euf.2016.11.002. Epub 2016 Nov 15. Eur Urol Focus. 2018. PMID: 28753806
-
Detection Rates of PSMA-PET Radiopharmaceuticals in Recurrent Prostate Cancer: A Systematic Review.Diagnostics (Basel). 2025 May 13;15(10):1224. doi: 10.3390/diagnostics15101224. Diagnostics (Basel). 2025. PMID: 40428217 Free PMC article. Review.
Cited by
-
Update on PSMA-based Prostate Cancer Imaging.Semin Nucl Med. 2024 Nov;54(6):941-950. doi: 10.1053/j.semnuclmed.2024.10.004. Epub 2024 Oct 28. Semin Nucl Med. 2024. PMID: 39490335 Review.
References
-
- Padhani AR, Lecouvet FE, Tunariu N, et al. . Rationale for modernising imaging in advanced prostate cancer. Eur Urol Focus. 2017;3:223–239. - PubMed
-
- Schaeffer EM, Srinivas S, Adra N, et al. . Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21:1067–1096. - PubMed
-
- Garje R, Rumble RB, Parikh RA, et al. . Systemic therapy update on 177lutetium-PSMA-617 for metastatic castration-resistant prostate cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2023;11:JCO2302128. - PubMed
-
- Mottet N, Cornford P, van den Bergh RCN, et al. . EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. EAU website. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-E.... Updated 2023. Accessed April 19, 2024.
-
- Highlights of prescribing information: POSLUMA (flotufolastat F 18) injection. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216023s000lbl.pdf. Revised May 2023. Accessed April 19, 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
