Bronchiectasis management in adults: state of the art and future directions
- PMID: 38782469
- PMCID: PMC11211698
- DOI: 10.1183/13993003.00518-2024
Bronchiectasis management in adults: state of the art and future directions
Abstract
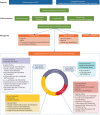
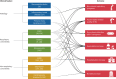
Formerly regarded as a rare disease, bronchiectasis is increasingly recognised. A renewed interest in this disease has led to significant progress in bronchiectasis research. Randomised clinical trials (RCTs) have demonstrated the benefits of airway clearance techniques, inhaled antibiotics and long-term macrolide therapy in bronchiectasis patients. However, the heterogeneity of bronchiectasis remains one of the most challenging aspects of management. Phenotypes and endotypes of bronchiectasis have been identified to help find "treatable traits" and partially overcome disease complexity. The goals of therapy for bronchiectasis are to reduce the symptom burden, improve quality of life, reduce exacerbations and prevent disease progression. We review the pharmacological and non-pharmacological treatments that can improve mucociliary clearance, reduce airway inflammation and tackle airway infection, the key pathophysiological features of bronchiectasis. There are also promising treatments in development for the management of bronchiectasis, including novel anti-inflammatory therapies. This review provides a critical update on the management of bronchiectasis focusing on treatable traits and recent RCTs.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: H. Choi reports grants from the Basic Science Research Program of the Korean Ministry of Education (grant number 2021R1I1A3052416), and consulting and lecture fees from Boryung Pharmaceutical Co., Ltd and Kolon Pharma. P.J. McShane reports study funding (paid to institution) from Boehringer Ingelheim, Armata, Paratek, Renovian, Spero, AN2 Therapeutics and Insmed, and speaker fees from Insmed. S. Aliberti reports grants or contracts from Insmed Incorporated, Chiesi, Fisher & Paykel and GlaxoSmithKline, royalties or licences from McGraw Hill, consulting fees from Insmed Incorporated, Insmed Italy, Insmed Ireland Ltd, Zambon SpA, AstraZeneca UK Ltd, AstraZeneca Pharmaceutical LP, CSL Behring GmbH, Grifols, Fondazione Internazionale Menarini, Moderna, Chiesi, MSD Italia Srl, Brahms, Physioassist SAS and GlaxoSmithKline SpA, payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline SpA, Thermofisher Scientific, Insmed Italy, Insmed Ireland, Zambon and Fondazione Internazionale Menarini, and participation on a data safety monitoring board or advisory board for Insmed Incorporated, Insmed Italy, AstraZeneca UK Ltd and MSD Italia Srl. J.D. Chalmers reports grants or contracts from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed, LifeArc and Novartis, and consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, Insmed, Grifols, Novartis, Boehringer Ingelheim, Pfizer, Janssen, Antabio and Zambon.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
