Swallowed topical steroid therapy for eosinophilic oesophagitis in children: practical, evidence-based guidance by the BSPGHAN Eosinophilic Oesophagitis Working Group
- PMID: 38782481
- PMCID: PMC11116858
- DOI: 10.1136/bmjpo-2023-002467
Swallowed topical steroid therapy for eosinophilic oesophagitis in children: practical, evidence-based guidance by the BSPGHAN Eosinophilic Oesophagitis Working Group
Abstract
Objective: To develop evidence-based guidance for topical steroid use in paediatric eosinophilic oesophagitis (pEoE) in the UK for both induction and maintenance treatment.
Methods: A systematic literature review using Cochrane guidance was carried out by the British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) Eosinophilic Oesophagitis (EoE) Working Group (WG) and research leads to determine the evidence base for preparation, dosing and duration of use of swallowed topical steroid (STS) formulations in EoE. Seven themes relating to pEoE were reviewed by the WG, alongside the Cochrane review this formed the evidence base for consensus recommendations for pEoE in the UK. We provide an overview of practical considerations including treatment regimen and dosing. Oral viscous budesonide (OVB) and, if agreed by local regulatory committees, orodispersible budesonide (budesonide 1 mg tablets) were selected for ease of use and with most improvement in histology. A practical 'how to prepare and use' OVB appendix is included. Side effects identified included candidiasis and adrenal gland suppression. The use of oral systemic steroids in strictures is discussed briefly.
Results: 2638 citations were identified and 18 randomised controlled trials were included. Evidence exists for the use of STS for induction and maintenance therapy in EoE, especially regarding histological improvement. Using the Appraisal of Guidelines, Research and Evaluation criteria, dosing of steroids by age (0.5 mg two times per day <10 years and 1 mg two times per day ≥10 years) for induction of at least 3 months was suggested based on evidence and practical consideration. Once histological remission is achieved, maintenance dosing of steroids appears to reduce the frequency and severity of relapse, as such a maintenance weaning regimen is proposed.
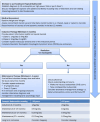
Conclusion: A practical, evidence-based flow chart and guidance recommendations with consensus from the EoE WG and education and research representatives of BSPGHAN were developed with detailed practical considerations for use in the UK.
Keywords: Child Health; Gastroenterology; Therapeutics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MKHA has received research grants and honorariums from BSPGHAN, Guts UK, Dr Falk Pharma, Mead Johnson, Abbott and Nutricia; AC has received grant funding from AZ, Bristol Myers Squibb, AVIR, Allakos, Dr Falk Pharma and Sanofi/Regeneron, and advisory panel honorarium was received from AZ, Sanofi/Regeneron, AVIR and Dr Falk Pharma; Guts UK Charity receives funding for the Falk Awards from Dr Falk Pharma.
Figures

References
-
- Straumann A, Spichtin HP, Bernoulli R, et al. . Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr 1994;124:1419–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
