SARS-CoV-2, periodontal pathogens, and host factors: The trinity of oral post-acute sequelae of COVID-19
- PMID: 38782605
- PMCID: PMC11260190
- DOI: 10.1002/rmv.2543
SARS-CoV-2, periodontal pathogens, and host factors: The trinity of oral post-acute sequelae of COVID-19
Abstract
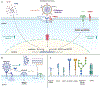
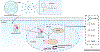
COVID-19 as a pan-epidemic is waning but there it is imperative to understand virus interaction with oral tissues and oral inflammatory diseases. We review periodontal disease (PD), a common inflammatory oral disease, as a driver of COVID-19 and oral post-acute-sequelae conditions (PASC). Oral PASC identifies with PD, loss of teeth, dysgeusia, xerostomia, sialolitis-sialolith, and mucositis. We contend that PD-associated oral microbial dysbiosis involving higher burden of periodontopathic bacteria provide an optimal microenvironment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. These pathogens interact with oral epithelial cells activate molecular or biochemical pathways that promote viral adherence, entry, and persistence in the oral cavity. A repertoire of diverse molecules identifies this relationship including lipids, carbohydrates and enzymes. The S protein of SARS-CoV-2 binds to the ACE2 receptor and is activated by protease activity of host furin or TRMPSS2 that cleave S protein subunits to promote viral entry. However, PD pathogens provide additional enzymatic assistance mimicking furin and augment SARS-CoV-2 adherence by inducing viral entry receptors ACE2/TRMPSS, which are poorly expressed on oral epithelial cells. We discuss the mechanisms involving periodontopathogens and host factors that facilitate SARS-CoV-2 infection and immune resistance resulting in incomplete clearance and risk for 'long-haul' oral health issues characterising PASC. Finally, we suggest potential diagnostic markers and treatment avenues to mitigate oral PASC.
Keywords: PASC; SARS‐CoV‐2; oral biology; oral microbiome; oral mucosal immunity; oral virology; ‘red complex’.
© 2024 The Author(s). Reviews in Medical Virology published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Figures

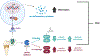
Similar articles
-
Oral SARS-CoV-2 Infection and Risk for Long Covid.Rev Med Virol. 2025 Mar;35(2):e70029. doi: 10.1002/rmv.70029. Rev Med Virol. 2025. PMID: 40074704 Free PMC article. Review.
-
Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19.J Genet. 2021;100(1):12. doi: 10.1007/s12041-021-01262-w. J Genet. 2021. PMID: 33707363 Free PMC article. Review.
-
Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration.Elife. 2020 Oct 26;9:e61552. doi: 10.7554/eLife.61552. Elife. 2020. PMID: 33103998 Free PMC article.
-
In Silico, In Vitro and In Cellulo Models for Monitoring SARS-CoV-2 Spike/Human ACE2 Complex, Viral Entry and Cell Fusion.Viruses. 2021 Feb 25;13(3):365. doi: 10.3390/v13030365. Viruses. 2021. PMID: 33669132 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
Cited by
-
Oral SARS-CoV-2 Infection and Risk for Long Covid.Rev Med Virol. 2025 Mar;35(2):e70029. doi: 10.1002/rmv.70029. Rev Med Virol. 2025. PMID: 40074704 Free PMC article. Review.
-
The Role of Dental-derived Stem Cell-based Therapy and Their Derived Extracellular Vesicles in Post-COVID-19 Syndrome-induced Tissue Damage.Stem Cell Rev Rep. 2024 Nov;20(8):2062-2103. doi: 10.1007/s12015-024-10770-y. Epub 2024 Aug 16. Stem Cell Rev Rep. 2024. PMID: 39150646 Review.
-
Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147.Int J Mol Sci. 2025 Jul 9;26(14):6583. doi: 10.3390/ijms26146583. Int J Mol Sci. 2025. PMID: 40724833 Free PMC article.
References
-
- Ohara-Nemoto Y, Shimoyama Y, Nakasato M, et al. Distribution of dipeptidyl peptidase (DPP) 4, DPP5, DPP7, and DPP11 in human oral microbiota – potent biomarkers indicating presence of periodontopathic bacteria. FEMS Microbiology Letters. Published online September 10, 2018. doi:10.1093/femsle/fny221 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

