METTL7A-mediated m6A modification of corin reverses bisphosphonates-impaired osteogenic differentiation of orofacial BMSCs
- PMID: 38782892
- PMCID: PMC11116408
- DOI: 10.1038/s41368-024-00303-1
METTL7A-mediated m6A modification of corin reverses bisphosphonates-impaired osteogenic differentiation of orofacial BMSCs
Abstract
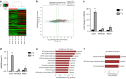
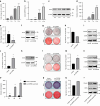
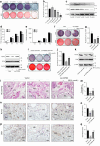
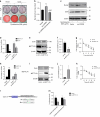
Bisphosphonate-related osteonecrosis of jaw (BRONJ) is characterized by impaired osteogenic differentiation of orofacial bone marrow stromal cells (BMSCs). Corin has recently been demonstrated to act as a key regulator in bone development and orthopedic disorders. However, the role of corin in BRONJ-related BMSCs dysfunction remains unclarified. A m6A epitranscriptomic microarray study from our group shows that the CORIN gene is significantly upregulated and m6A hypermethylated during orofacial BMSCs osteogenic differentiation. Corin knockdown inhibits BMSCs osteogenic differentiation, whereas corin overexpression or soluble corin (sCorin) exerts a promotion effect. Furthermore, corin expression is negatively regulated by bisphosphonates (BPs). Corin overexpression or sCorin reverses BPs-impaired BMSCs differentiation ability. Mechanistically, we find altered expression of phos-ERK in corin knockdown/overexpression BMSCs and BMSCs under sCorin stimulation. PD98059 (a selective ERK inhibitor) blocks the corin-mediated promotion effect. With regard to the high methylation level of corin during osteogenic differentiation, we apply a non-selective m6A methylase inhibitor, Cycloleucine, which also blocks the corin-mediated promotion effect. Furthermore, we demonstrate that METTL7A modulates corin m6A modification and reverses BPs-impaired BMSCs function, indicating that METTL7A regulates corin expression and thus contributes to orofacial BMSCs differentiation ability. To conclude, our study reveals that corin reverses BPs-induced BMSCs dysfunction, and METTL7A-mediated corin m6A modification underlies corin promotion of osteogenic differentiation via the ERK pathway. We hope this brings new insights into future clinical treatments for BRONJ.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Fliefel R, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int. J. Oral Maxillofac Surg. 2015;44:568–585. doi: 10.1016/j.ijom.2015.01.026. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

