Single molecule delivery into living cells
- PMID: 38782907
- PMCID: PMC11116494
- DOI: 10.1038/s41467-024-48608-3
Single molecule delivery into living cells
Abstract
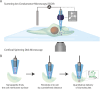
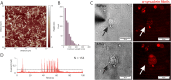
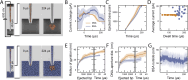
Controlled manipulation of cultured cells by delivery of exogenous macromolecules is a cornerstone of experimental biology. Here we describe a platform that uses nanopipettes to deliver defined numbers of macromolecules into cultured cell lines and primary cells at single molecule resolution. In the nanoinjection platform, the nanopipette is used as both a scanning ion conductance microscope (SICM) probe and an injection probe. The SICM is used to position the nanopipette above the cell surface before the nanopipette is inserted into the cell into a defined location and to a predefined depth. We demonstrate that the nanoinjection platform enables the quantitative delivery of DNA, globular proteins, and protein fibrils into cells with single molecule resolution and that delivery results in a phenotypic change in the cell that depends on the identity of the molecules introduced. Using experiments and computational modeling, we also show that macromolecular crowding in the cell increases the signal-to-noise ratio for the detection of translocation events, thus the cell itself enhances the detection of the molecules delivered.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

