Transcriptomic analysis of subarachnoid cysts of Taenia solium reveals mechanisms for uncontrolled proliferation and adaptations to the microenvironment
- PMID: 38782926
- PMCID: PMC11116493
- DOI: 10.1038/s41598-024-61973-9
Transcriptomic analysis of subarachnoid cysts of Taenia solium reveals mechanisms for uncontrolled proliferation and adaptations to the microenvironment
Abstract
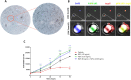
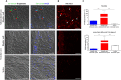
Subarachnoid neurocysticercosis (SANCC) is caused by an abnormally transformed form of the metacestode or larval form of the tapeworm Taenia solium. In contrast to vesicular parenchymal and ventricular located cysts that contain a viable scolex and are anlage of the adult tapeworm, the subarachnoid cyst proliferates to form aberrant membranous cystic masses within the subarachnoid spaces that cause mass effects and acute and chronic arachnoiditis. How subarachnoid cyst proliferates and interacts with the human host is poorly understood, but parasite stem cells (germinative cells) likely participate. RNA-seq analysis of the subarachnoid cyst bladder wall compared to the bladder wall and scolex of the vesicular cyst revealed that the subarachnoid form exhibits activation of signaling pathways that promote proliferation and increased lipid metabolism. These adaptions allow growth in a nutrient-limited cerebral spinal fluid. In addition, we identified therapeutic drug targets that would inhibit growth of the parasite, potentially increase effectiveness of treatment, and shorten its duration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

