TET activity safeguards pluripotency throughout embryonic dormancy
- PMID: 38783076
- PMCID: PMC11479945
- DOI: 10.1038/s41594-024-01313-7
TET activity safeguards pluripotency throughout embryonic dormancy
Abstract
Dormancy is an essential biological process for the propagation of many life forms through generations and stressful conditions. Early embryos of many mammals are preservable for weeks to months within the uterus in a dormant state called diapause, which can be induced in vitro through mTOR inhibition. Cellular strategies that safeguard original cell identity within the silent genomic landscape of dormancy are not known. Here we show that the protection of cis-regulatory elements from silencing is key to maintaining pluripotency in the dormant state. We reveal a TET-transcription factor axis, in which TET-mediated DNA demethylation and recruitment of methylation-sensitive transcription factor TFE3 drive transcriptionally inert chromatin adaptations during dormancy transition. Perturbation of TET activity compromises pluripotency and survival of mouse embryos under dormancy, whereas its enhancement improves survival rates. Our results reveal an essential mechanism for propagating the cellular identity of dormant cells, with implications for regeneration and disease.
© 2024. The Author(s).
Conflict of interest statement
I.A.I. and T.A. are inventors on a patent application (no. EP3325621B1, European Patent Office) regarding the s-oligo design used in FLASH experiments. The other authors declare no competing interests.
Figures


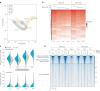

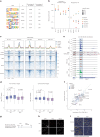



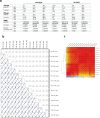



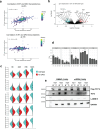



References
-
- Renfree, M. B. & Fenelon, J. C. The enigma of embryonic diapause. Development144, 3199–3210 (2017). - PubMed
-
- Fu, Z. et al. Integral proteomic analysis of blastocysts reveals key molecular machinery governing embryonic diapause and reactivation for implantation in mice. Biol. Reprod.90, 1–11 (2014). Article 52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

