The tardigrade-derived mitochondrial abundant heat soluble protein improves adipose-derived stem cell survival against representative stressors
- PMID: 38783150
- PMCID: PMC11116449
- DOI: 10.1038/s41598-024-62693-w
The tardigrade-derived mitochondrial abundant heat soluble protein improves adipose-derived stem cell survival against representative stressors
Abstract
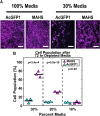
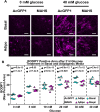
Human adipose-derived stem cell (ASC) grafts have emerged as a powerful tool in regenerative medicine. However, ASC therapeutic potential is hindered by stressors throughout their use. Here we demonstrate the transgenic expression of the tardigrade-derived mitochondrial abundant heat soluble (MAHS) protein for improved ASC resistance to metabolic, mitochondrial, and injection shear stress. In vitro, MAHS-expressing ASCs demonstrate up to 61% increased cell survival following 72 h of incubation in phosphate buffered saline containing 20% media. Following up to 3.5% DMSO exposure for up to 72 h, a 14-49% increase in MAHS-expressing ASC survival was observed. Further, MAHS expression in ASCs is associated with up to 39% improved cell viability following injection through clinically relevant 27-, 32-, and 34-gauge needles. Our results reveal that MAHS expression in ASCs supports survival in response to a variety of common stressors associated with regenerative therapies, thereby motivating further investigation into MAHS as an agent for stem cell stress resistance. However, differentiation capacity in MAHS-expressing ASCs appears to be skewed in favor of osteogenesis over adipogenesis. Specifically, activity of the early bone formation marker alkaline phosphatase is increased by 74% in MAHS-expressing ASCs following 14 days in osteogenic media. Conversely, positive area of the neutral lipid droplet marker BODIPY is decreased by up to 10% in MAHS-transgenic ASCs following 14 days in adipogenic media. Interestingly, media supplementation with up to 40 mM glucose is sufficient to restore adipogenic differentiation within 14 days, prompting further analysis of mechanisms underlying interference between MAHS and differentiation processes.
Keywords: Injections; Stem cells; Stress tolerance; Tardigrade; Transgenic.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells.J Transl Med. 2016 May 6;14(1):126. doi: 10.1186/s12967-016-0881-1. J Transl Med. 2016. PMID: 27153799 Free PMC article.
-
Aspirin Inhibits the In Vitro Adipogenic Differentiation of Human Adipose Tissue-Derived Stem Cells in a Dose-Dependent Manner.Int J Mol Sci. 2025 Jan 20;26(2):853. doi: 10.3390/ijms26020853. Int J Mol Sci. 2025. PMID: 39859567 Free PMC article.
-
The role of serum amyloid A1 in the adipogenic differentiation of human adipose-derived stem cells basing on single-cell RNA sequencing analysis.Stem Cell Res Ther. 2022 May 7;13(1):187. doi: 10.1186/s13287-022-02873-5. Stem Cell Res Ther. 2022. PMID: 35525990 Free PMC article.
-
Novel mitochondria-targeted heat-soluble proteins identified in the anhydrobiotic Tardigrade improve osmotic tolerance of human cells.PLoS One. 2015 Feb 12;10(2):e0118272. doi: 10.1371/journal.pone.0118272. eCollection 2015. PLoS One. 2015. PMID: 25675104 Free PMC article.
-
Human Adipose-Derived Mesenchymal Stromal/Stem Cell Spheroids Possess High Adipogenic Capacity and Acquire an Adipose Tissue-like Extracellular Matrix Pattern.Tissue Eng Part A. 2020 Aug;26(15-16):915-926. doi: 10.1089/ten.TEA.2019.0206. Epub 2020 Apr 9. Tissue Eng Part A. 2020. PMID: 32070231
Cited by
-
Life on the dry side: a roadmap to understanding desiccation tolerance and accelerating translational applications.Nat Commun. 2025 Apr 6;16(1):3284. doi: 10.1038/s41467-025-58656-y. Nat Commun. 2025. PMID: 40189591 Free PMC article. Review.
-
Expressing intrinsically-disordered tardigrade proteins has positive effects on acute but not chronic stress tolerance in Saccharomyces cerevisiae.PLoS One. 2025 Jun 6;20(6):e0325682. doi: 10.1371/journal.pone.0325682. eCollection 2025. PLoS One. 2025. PMID: 40478923 Free PMC article.
References
-
- Stem Cell Therapy Market (By Product: Adult Stem Cells (ASCs), Human Embryonic Stem Cells (HESCs), Induced Pluripotent Stem Cells (iPSCs), Very Small Embryonic Like Stem Cells; By Therapy Type; By Application; By Technology; By End User)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022–2030. https://www.precedenceresearch.com/stem-cell-therapy-market. Accessed 17 Aug 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

