MERTK in the rat trigeminal system: a potential novel target for cluster headache?
- PMID: 38783191
- PMCID: PMC11119394
- DOI: 10.1186/s10194-024-01791-6
MERTK in the rat trigeminal system: a potential novel target for cluster headache?
Abstract
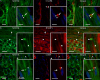
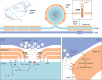
The trigeminal system is key to the pathophysiology of migraine and cluster headache, two primary headache disorders that share many features. Recently, MER proto-oncogene tyrosine kinase (MERTK), a cell surface receptor, was strongly associated with cluster headache through genetic studies. Further, the MERTK ligand galectin-3 has been found to be elevated in serum of migraine patients. In this study, MERTK and MERTK ligands were investigated in key tissue to better understand their potential implication in the pathophysiology of primary headache disorders. Immunohistochemistry was used to map MERTK and galectin-3 expression in rat trigeminal ganglia. RT-qPCR was used to assess MERTK gene expression in blood, and ELISA immunoassays were used for MERTK ligand quantification in serum from study participants with and without cluster headache. MERTK gene expression was elevated in blood samples from study participants with cluster headache compared to controls. In addition, MERTK ligand galectin-3 was found at increased concentration in the serum of study participants with cluster headache, whereas the levels of MERTK ligands growth arrest specific 6 and protein S unaffected. MERTK and galectin-3 were both expressed in rat trigeminal ganglia. Galectin-3 was primarily localized in smaller neurons and to a lesser extent in C-fibres, while MERTK was found in satellite glia cells and in the outer membrane of Schwann cells. Interestingly, a strong MERTK signal was found specifically in the region proximal to the nodes of Ranvier. The overexpression of MERTK and galectin-3 in tissue from study participants with cluster headache, as well as the presence of MERTK in rat peripheral satellite glia cells and Schwann cells in the trigeminal ganglia, further highlights MERTK signalling as an interesting potential future therapeutic target in primary headache.
Keywords: GWAS; Galectin-3; MER proto-oncogene tyrosine kinase; Migraine; Trigeminal system.
© 2024. The Author(s).
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Nesbitt AD, Goadsby PJ (2012) Cluster headache. BMJ. 344 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

