Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i
- PMID: 38783218
- PMCID: PMC11112888
- DOI: 10.1186/s12885-024-12269-8
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i
Abstract
Background: Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the European Society for Medical Oncology (ESMO) guidelines as the first-line (1 L) treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, locally advanced/metastatic breast cancer (HR+/HER2- LABC/mBC). Although there are many treatment options, there is no clear standard of care for patients following 1 L CDK4/6i. Understanding the real-world effectiveness of subsequent therapies may help to identify an unmet need in this patient population. This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC.
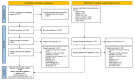
Methods: MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022. Grey literature was searched to identify relevant conference abstracts published from 2019 to 2022. The review was conducted in accordance with PRISMA guidelines (PROSPERO registration: CRD42023383914). Data were qualitatively synthesized and weighted average median real-world progression-free survival (rwPFS) was calculated for NCCN/ESMO-recommended post-1 L CDK4/6i treatment regimens.
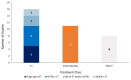
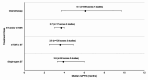
Results: Twenty records (9 full-text articles and 11 conference abstracts) encompassing 18 unique studies met the eligibility criteria and reported outcomes for second-line (2 L) treatments after 1 L CDK4/6i; no studies reported disaggregated outcomes in the third-line setting or beyond. Sixteen studies included NCCN/ESMO guideline-recommended treatments with the majority evaluating endocrine-based therapy; five studies on single-agent ET, six studies on mammalian target of rapamycin inhibitors (mTORi) ± ET, and three studies with a mix of ET and/or mTORi. Chemotherapy outcomes were reported in 11 studies. The most assessed outcome was median rwPFS; the weighted average median rwPFS was calculated as 3.9 months (3.3-6.0 months) for single-agent ET, 3.6 months (2.5-4.9 months) for mTORi ± ET, 3.7 months for a mix of ET and/or mTORi (3.0-4.0 months), and 6.1 months (3.7-9.7 months) for chemotherapy. Very few studies reported other effectiveness outcomes and only two studies reported safety outcomes. Most studies had heterogeneity in patient- and disease-related characteristics.
Conclusions: The real-world effectiveness of current 2 L treatments post-1 L CDK4/6i are suboptimal, highlighting an unmet need for this patient population.
Keywords: Breast cancer; First-line CDK4/6i; HR+/HER2-; Real-world evidence; Systematic literature review.
© 2024. The Author(s).
Conflict of interest statement
The authors of this manuscript declare that the research presented was funded by Pfizer Inc. and Arvinas. While the support from Pfizer Inc. and Arvinas was instrumental in facilitating this research, the authors affirm that their interpretation of the data and the content of this manuscript were conducted independently and without bias to maintain the transparency and integrity of the research. IAS, SK, BH, and BN are employees of EVERSANA, Canada, which was a paid consultant to Pfizer in connection with the development of this manuscript.
Figures
Similar articles
-
Real-world evidence from Japan regarding survival outcomes and treatment sequence in patients receiving CDK4/6 inhibitor plus endocrine therapy as first- or second-line treatment for hormone receptor-positive, HER2-negative advanced or metastatic breast cancer.Breast Cancer. 2025 Jul;32(4):841-856. doi: 10.1007/s12282-025-01713-7. Epub 2025 May 20. Breast Cancer. 2025. PMID: 40392524 Free PMC article.
-
Disparities in receipt of 1-st line CDK4/6 inhibitors with endocrine therapy for treatment of hormone receptor positive, HER2 negative metastatic breast cancer in the real-world setting.Breast Cancer Res. 2024 Oct 18;26(1):144. doi: 10.1186/s13058-024-01902-w. Breast Cancer Res. 2024. PMID: 39425174 Free PMC article.
-
First- vs second-line CDK 4/6 inhibitor use for patients with hormone receptor positive, human epidermal growth-factor receptor-2 negative, metastatic breast cancer in the real world setting.Breast Cancer Res Treat. 2024 Nov;208(2):263-273. doi: 10.1007/s10549-024-07415-6. Epub 2024 Jun 26. Breast Cancer Res Treat. 2024. PMID: 38922546 Free PMC article.
-
Efficacy of Subsequent Treatments After Disease Progression on CDK4/6 Inhibitors in Patients With Hormone Receptor-Positive Advanced Breast Cancer.JCO Oncol Pract. 2025 Jun;21(6):832-842. doi: 10.1200/OP-24-00649. Epub 2024 Dec 17. JCO Oncol Pract. 2025. PMID: 39689274
-
CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies.Future Oncol. 2021 Jun;17(16):2107-2122. doi: 10.2217/fon-2020-1264. Epub 2021 Mar 5. Future Oncol. 2021. PMID: 33663223
Cited by
-
Conditional progression-free survival in patients with metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with first-line ribociclib and endocrine therapy: real-world data from the RIBANNA study.ESMO Open. 2025 Jun;10(6):105105. doi: 10.1016/j.esmoop.2025.105105. Epub 2025 May 16. ESMO Open. 2025. PMID: 40381382 Free PMC article.
-
Effect of Tasurgratinib as an Orally Available FGFR1-3 Inhibitor on Resistance to a CDK4/6 Inhibitor and Endocrine Therapy in ER+/HER2- Breast Cancer Preclinical Models.Cancers (Basel). 2025 Mar 24;17(7):1084. doi: 10.3390/cancers17071084. Cancers (Basel). 2025. PMID: 40227585 Free PMC article.
-
Treatment Sequencing in Metastatic HR+/HER2- Breast Cancer: A Delphi Consensus.Cancers (Basel). 2025 Apr 23;17(9):1412. doi: 10.3390/cancers17091412. Cancers (Basel). 2025. PMID: 40361341 Free PMC article. Review.
References
-
- World Health Organization (WHO). Breast Cancer Facts Sheet [updated July 12 2023. https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
-
- Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524– 41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

