Efficacy of intrathecal mesenchymal stem cell-neural progenitor therapy in progressive MS: results from a phase II, randomized, placebo-controlled clinical trial
- PMID: 38783390
- PMCID: PMC11119709
- DOI: 10.1186/s13287-024-03765-6
Efficacy of intrathecal mesenchymal stem cell-neural progenitor therapy in progressive MS: results from a phase II, randomized, placebo-controlled clinical trial
Abstract
Background: Mesenchymal stem cell-neural progenitors (MSC-NPs) are a bone marrow mesenchymal stem cell (MSC)-derived ex vivo manipulated cell product with therapeutic potential in multiple sclerosis (MS). The objective of this study was to determine efficacy of intrathecal (IT) MSC-NP treatment in patients with progressive MS.
Methods: The study is a phase II randomized, double-blind, placebo-controlled clinical trial with a compassionate crossover design conducted at a single site. Subjects were stratified according to baseline Expanded Disability Status Scale (EDSS) (3.0-6.5) and disease subtype (secondary or primary progressive MS) and randomized into either treatment or placebo group to receive six IT injections of autologous MSC-NPs or saline every two months. The primary outcome was EDSS Plus, defined by improvement in EDSS, timed 25-foot walk (T25FW) or nine-hole peg test. Secondary outcomes included the individual components of EDSS Plus, the six-minute walk test (6MWT), urodynamics testing, and brain atrophy measurement.
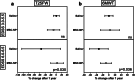
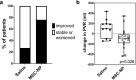
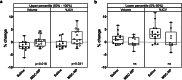
Results: Subjects were randomized into MSC-NP (n = 27) or saline (n = 27) groups. There was no difference in EDSS Plus improvement between the MSC-NP (33%) and saline (37%) groups. Exploratory subgroup analysis demonstrated that in subjects who require assistance for ambulation (EDSS 6.0-6.5) there was a significantly higher percentage of improvement in T25FW and 6MWT in the MSC-NP group (3.7% ± 23.1% and - 9.2% ± 18.2%) compared to the saline group (-54.4% ± 70.5% and - 32.1% ± 30.0%), (p = 0.030 and p = 0.036, respectively). IT-MSC-NP treatment was also associated with improved bladder function and reduced rate of grey matter atrophy on brain MRI. Biomarker analysis demonstrated increased MMP9 and decreased CCL2 levels in the cerebrospinal fluid following treatment.
Conclusion: Results from exploratory outcomes suggest that IT-MSC-NP treatment may be associated with a therapeutic response in a subgroup of MS patients.
Trial registration: ClinicalTrials.gov NCT03355365, registered November 14, 2017, https://clinicaltrials.gov/study/NCT03355365?term=NCT03355365&rank=1 .
Keywords: Cell therapy; Clinical trial; Intrathecal; MSC-NP; Mesenchymal stem cell; Multiple sclerosis; Progressive multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
VH and SS are listed as inventors on a US patent issued to the Tisch MS Research Center of New York and is considered a non-financial competing interest. All other authors declare no competing interests.
Figures

References
-
- Correale J, Gaitan MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017;140(3):527–46. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

