Biomarker endpoints in cancer cachexia clinical trials: Systematic Review 5 of the cachexia endpoint series
- PMID: 38783477
- PMCID: PMC11154797
- DOI: 10.1002/jcsm.13491
Biomarker endpoints in cancer cachexia clinical trials: Systematic Review 5 of the cachexia endpoint series
Abstract
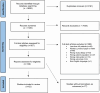
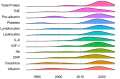
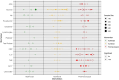
Regulatory agencies require evidence that endpoints correlate with clinical benefit before they can be used to approve drugs. Biomarkers are often considered surrogate endpoints. In cancer cachexia trials, the measurement of biomarkers features frequently. The aim of this systematic review was to assess the frequency and diversity of biomarker endpoints in cancer cachexia trials. A comprehensive electronic literature search of MEDLINE, Embase and Cochrane (1990-2023) was completed. Eligible trials met the following criteria: adults (≥18 years), prospective design, more than 40 participants, use of a cachexia intervention for more than 14 days and use of a biomarker(s) as an endpoint. Biomarkers were defined as any objective measure that was assayed from a body fluid, including scoring systems based on these assays. Routine haematology and biochemistry to monitor intervention toxicity were not considered. Data extraction was performed using Covidence, and reporting followed PRISMA guidance (PROSPERO: CRD42022276710). A total of 5975 studies were assessed, of which 52 trials (total participants = 6522) included biomarkers as endpoints. Most studies (n = 29, 55.7%) included a variety of cancer types. Pharmacological interventions (n = 27, 51.9%) were most evaluated, followed by nutritional interventions (n = 20, 38.4%). Ninety-nine different biomarkers were used across the trials, and of these, 96 were assayed from blood. Albumin (n = 29, 55.8%) was assessed most often, followed by C-reactive protein (n = 22, 42.3%), interleukin-6 (n = 16, 30.8%) and tumour necrosis factor-α (n = 14, 26.9%), the latter being the only biomarker that was used to guide sample size calculations. Biomarkers were explicitly listed as a primary outcome in six trials. In total, 12 biomarkers (12.1% of 99) were used in six trials or more. Insulin-like growth factor binding protein 3 (IGFBP-3) and insulin-like growth factor 1 (IGF-1) levels both increased significantly in all three trials in which they were both used. This corresponded with a primary outcome, lean body mass, and was related to the pharmacological mechanism. Biomarkers were predominately used as exploratory rather than primary endpoints. The most commonly used biomarker, albumin, was limited by its lack of responsiveness to nutritional intervention. For a biomarker to be responsive to change, it must be related to the mechanism of action of the intervention and/or the underlying cachexia process that is modified by the intervention, as seen with IGFBP-3, IGF-1 and anamorelin. To reach regulatory approval as an endpoint, the relationship between the biomarker and clinical benefit must be clarified.
Keywords: biomarkers; cachexia; cancer; endpoints; trials.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
SDA has received grants and personal fees from Vifor and Abbott Vascular and personal fees for consultancies, trial committee work and/or lectures from Actimed, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BioVentrix, Brahms, Cardiac Dimensions, Cardior, Cordio, CVRx, Cytokinetics, Edwards, Farraday Pharmaceuticals, GSK, HeartKinetics, Impulse Dynamics, Novartis, Occlutech, Pfizer, Repairon, Sensible Medical, Servier, Vectorious and V‐Wave. He is the co‐inventor of two patent applications regarding MR‐proANP (DE 102007010834 and DE 102007022367), but he does not personally benefit from the related issued patents. JA has received personal fees from Danone. MF has received personal fees from Pfizer. MJH has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETS and NIHR. MJH has consulted for and is a member of the Achilles Therapeutics Scientific Advisory Board and Steering Committee. MJH has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster and Bristol Myers Squibb and is a co‐inventor on a European patent application relating to methods to detect lung cancer (PCT/US2017/028013). BJAL has received personal fees for consulting from Artelo, Actimed, Faraday, Kyona Kirin and Toray. RJES has received personal fees for consulting from Artelo, Actimed, Faraday and Helsinn. EJR has a consulting/advisory role for Napo, AIM Specialty Health, Oragenics, BASF, Immuneering, Vector Oncology, Asahi Kasei, Heron, Pfizer/EMD Serono and Mitobridge.
Figures
References
-
- Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. - PubMed
-
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- McMillan DC. An inflammation‐based prognostic score and its role in the nutrition‐based management of patients with cancer. Proc Nutr Soc 2008;67:257–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

