HPTN 083-02: factors influencing adherence to injectable PrEP and retention in an injectable PrEP study
- PMID: 38783534
- PMCID: PMC11116478
- DOI: 10.1002/jia2.26252
HPTN 083-02: factors influencing adherence to injectable PrEP and retention in an injectable PrEP study
Abstract
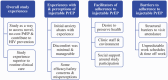
Introduction: HPTN 083 demonstrated the superiority of long-acting cabotegravir (CAB-LA) versus daily oral emtricitabine/tenofovir disoproxil fumarate (TDF/FTC) as pre-exposure prophylaxis (PrEP) among cisgender men and transgender women who have sex with men (MSM/TGW). HPTN 083 provided the first opportunity to understand experiences with injectable PrEP in a clinical trial.
Methods: Participants from two US sites (Chicago, IL and Atlanta, GA) and one international site (Rio de Janeiro, Brazil) were purposively sampled for individual qualitative interviews (N = 40), between November 2019 and March 2020, to explore trial experiences, barriers to adherence and other factors that may have impacted study implementation or outcomes. The blinded phase ended early due to efficacy; this analysis includes interviews conducted prior to unblinding with three groups defined by adherence (i.e. injection visit attendance): adherent (n = 27), non-adherent (n = 12) and early discontinuers (n = 1). Data were organized using NVivo software and analysed using content analysis.
Results: Participants (mean age: 27) were primarily cisgender MSM (90%) and Black/African American (60%). Reasons for trial enrolment and PrEP use included a preference for using HIV prevention medication versus treatment in the event of HIV acquisition; the ability to enhance health via study-related education and services; access to a novel, convenient HIV prevention product at no cost; and contributing to MSM/TGW communities through research. Participants contrasted positive experiences with study staff with their routine clinical care, and emphasized increased scheduling flexibility, thorough communication, non-judgemental counselling and open, affirming environments (e.g. compassion, less stigma) as adherence facilitators. Injection experiences were positive overall; some described early injection-related anxiety, which abated with time and when given some measure of control (e.g. pre-injection countdown), and minimal injection site discomfort. Some concerns and misperceptions about injectable PrEP were reported. Barriers to adherence, across all adherence categories, included structural factors (e.g. financial constraints, travel) and competing demands (e.g. work schedules).
Conclusions: Respondents viewed injectable PrEP trial participation as a positive experience and a means of enhancing wellbeing. Study site flexibility and affirming clinic environments, inclusive of non-judgemental counselling, were key facilitators of adherence. To support injection persistence, interventions that address structural barriers and promote flexible means of injection delivery may be most effective.
Trial registration: ClinicalTrials.gov NCT02720094.
Keywords: HIV prevention; injectable PrEP; men who have sex with men; pre‐exposure prophylaxis; qualitative; transgender women.
© 2024 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
JS received support from the National Institutes of Health for his work on this study. He is also a member of Merck KGaA's Ethics Advisory Panel and Stem Cell Research Oversight Committee; a member of IQVIA's Ethics Advisory Panel; a member of Aspen Neurosciences Clinical Advisory Panel; a member of a Merck Data Monitoring Committee; and a consultant to Biogen. None of these latter activities are related to the material discussed in this manuscript. CFK has received research grants to her institution from Gilead Sciences, ViiV Healthcare, Moderna, Novavax and Humanigen. RJL serves on a Scientific Advisory Board for Merck. ARR is an employee and shareholder of ViiV Healthcare. NP's institution received research funding from Gilead Sciences, consultant fees from Merck and advisory board fees from ViiV Healthcare (no direct payment to NP). All other authors declare that they have no competing interests.