Copper-Catalyzed Continuous-Flow Transfer Hydrogenation of Nitroarenes to Anilines: A Scalable and Reliable Protocol
- PMID: 38783856
- PMCID: PMC11110069
- DOI: 10.1021/acs.oprd.3c00144
Copper-Catalyzed Continuous-Flow Transfer Hydrogenation of Nitroarenes to Anilines: A Scalable and Reliable Protocol
Abstract
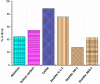
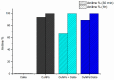
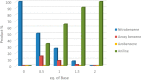
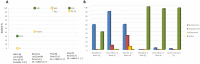
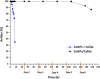
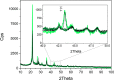
A robust supported catalyst that is made up of copper nanoparticles on Celite has been successfully prepared for the selective transfer hydrogenation of aromatic nitrobenzenes to anilines under continuous flow. The method is efficient and environmentally benign thanks to the absence of hydrogen gas and precious metals. Long-term stability studies show that the catalytic system is able to achieve very high nitrobenzene conversion (>99%) when working for up to 145 h. The versatility of the transfer hydrogenation system has been tested using representative examples of nitroarenes, with moderate-to-excellent yields being obtained. The packed bed reactor (PBR) permits the use of a setup that can provide products via simple isolation by SPE without the need for further purification. The recovery and reuse of either EG or the ion-exchange resin leads to consistent waste reduction; therefore, E-factor distribution analysis has highlighted the environmental efficiency of this synthetic protocol.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Gioiello A.; Mancino V.; Filipponi P.; Mostarda S.; Cerra B. Concepts and Optimization Strategies of Experimental Design in Continuous-Flow Processing. J. Flow Chem. 2016, 6 (3), 167–180. 10.1556/1846.2016.00012. - DOI
-
- Dallinger D.; Kappe C. O. Why flow means green – Evaluating the merits of continuous processing in the context of sustainability. Curr. Opin. Green Sustain. Chem. 2017, 7, 6–12. 10.1016/j.cogsc.2017.06.003. - DOI
LinkOut - more resources
Full Text Sources
